Chapter: Modern Analytical Chemistry: Uality Assurance
Quality Control
Quality Control
Quality control encompasses all activities used to bring
a system into statistical
control. The most important facet
of quality control
is a set of written
directives de- scribing all
relevant laboratory-specific,
technique-specific, sample-specific, method-specific, and protocol-specific operations. Good laboratory practices (GLPs) describe the general laboratory operations that need
to be followed in any analysis. These practices include
properly recording data and maintaining records, using chain-of-custody forms
for samples that are submitted for analysis, specifying and purifying chemical reagents,
preparing commonly used reagents, cleaning
and calibrating glassware, training
laboratory personnel, and maintaining the laboratory
facilities and general
laboratory equipment.
Good measurement practices (GMPs) describe operations specific to a tech- nique. In general, GMPs provide instructions for maintaining, calibrating, and using the equipment and instrumentation that form the basis for a specific tech- nique. For example, a GMP for a titration describes how to calibrate a buret (if necessary), how to fill a buret with the titrant, the correct way to read the volume of titrant in the buret, and the correct way to dispense the titrant.
The operations that
need to be performed when
analyzing a specific analyte in a specific matrix are defined
by a standard operations procedure (SOP). The
SOP describes all steps
taken during the analysis, including: how the sample
is processed in the laboratory, the analyte’s
separation from potential interferents, how the method is standardized, how the analytical signal is measured, how the data are
transformed into the desired result,
and the quality
assessment tools that will be used
to maintain quality
control. If the laboratory is responsible for sampling, then the
SOP will also
state how the
sample is to be collected and preserved and
the na- ture of any prelaboratory processing. A SOP may be developed and used by a single laboratory, or it may be a standard procedure approved by an organization such as
the American Society for Testing
and Materials or the Federal
Food and Drug
Ad- ministration. A typical
SOP is provided in the following example.
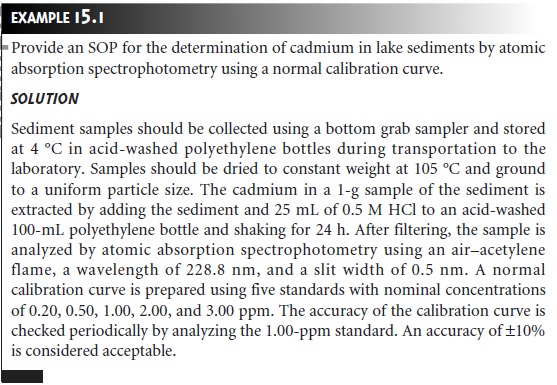
Although an SOP provides a written procedure, it is not necessary to follow the procedure exactly as long as any modifications are identified. On the other
hand, a protocol for a specific purpose (PSP), which is the most detailed
of the written quality control directives, must be followed
exactly if the results of the analysis
are to be accepted.
In many cases the required
elements of a PSP are established by the
agency sponsoring the analysis. For example, labs working under
contract with the Environmental Protection Agency must
develop a PSP
that addresses such
items as sampling and
sample custody, frequency of calibration, schedules for the preventive maintenance of equipment and
instrumentation, and management of the quality assurance program.
Two additional aspects
of a quality control program
deserve mention. The first
is the physical inspection of samples, measurements and results by the individuals responsible for collecting and analyzing the samples.1 For example, sediment sam- ples
might be screened
during collection, and samples containing “foreign objects,”
such as pieces of metal,
be discarded without
being analyzed. Samples
that are dis- carded can then be replaced with additional samples.
When a sudden change in the
performance of an instrument is observed, the analyst may choose to repeat those measurements that might be adversely influenced. The analyst may also decide to
reject a result and reanalyze the sample when the result
is clearly unreasonable. By identifying samples, measurements, and results that
may be subject
to gross errors, inspection helps control the quality of an analysis.
A final component of a quality
control program is the certification of an ana- lyst’s competence to perform
the analysis for which he or she is responsible.7 Before
an analyst is allowed to perform a new analytical method, he or she may
be required to successfully analyze an independent check sample with acceptable accuracy
and precision. The check
sample should be similar in composition to samples that the
analyst will routinely encounter, with a concentration that is 5 to 50 times that of
the method’s detection limit.
Related Topics