Chapter: Modern Analytical Chemistry: Uality Assurance
Quality Assessment
Quality Assessment
The written directives of a quality
control program are a necessary, but not a suffi-
cient, condition for
obtaining and maintaining an analysis in a state
of statistical control.
Although quality control directives explain how an analysis should be properly
conducted, they do not indicate
whether the system
is under statistical control. This
is the role
of quality assessment, which is the second
component of a quality assurance program.
The
goals
of quality
assessment are to determine
when a system has reached
a state of statistical control; to detect when the system has moved out of statistical control; and, if possible, to suggest
why a loss of statistical control has occurred so that corrective
ac- tions can be taken.
For convenience, the methods of quality
assessment are divided into two categories: internal methods that are coordinated within the laboratory and exter-
nal methods for which an outside agency or individual is responsible.
Internal Methods of Quality Assessment
The most useful
methods for quality
assessment are those
that are coordinated by the laboratory and that provide
the analyst with immediate feedback
about the sys- tem’s state of statistical control. Internal methods
of quality assessment included in this section
are the analysis
of duplicate samples,
the analysis of blanks, the analysis
of standard samples, and spike
recoveries.
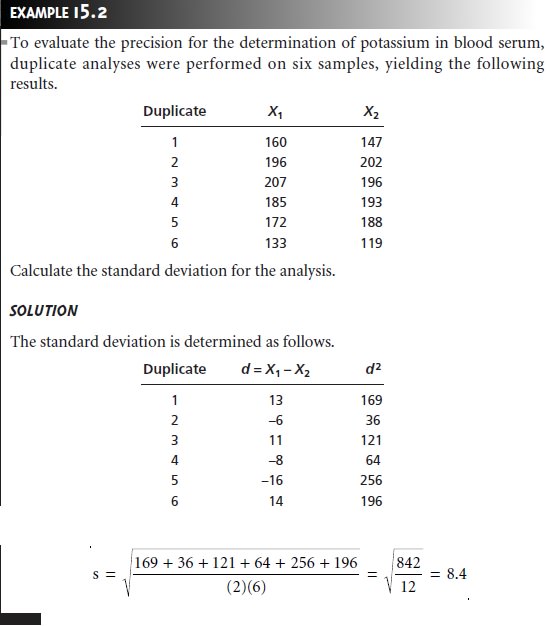
Analysis of Duplicate Samples
An effective method
for determining the precision
of an analysis is to analyze duplicate samples.
In most cases
the duplicate samples are taken from a single gross
sample (also called
a split sample),
although in some cases the duplicates must be
independently collected gross samples. The results from the duplicate samples,
X1 and X2, are evaluated by determining the difference,
d, or the
relative difference, (d)r, between
the samples
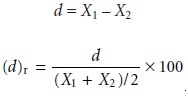
and comparing the results with accepted values,
such as those
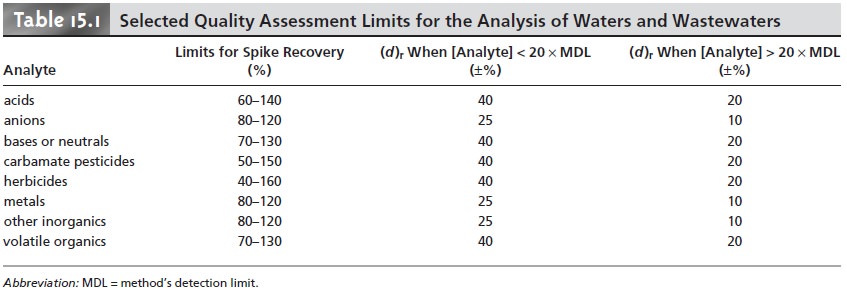
shown in Table
15.1 for the analysis
of waters and wastewaters.
Alternatively, the results
for a set of n duplicates are
combined to estimate the standard deviation for the analysis
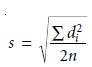
where di is the difference between the ith pair of duplicates. The degrees of freedom
for the standard deviation is the same as the number of duplicate samples.
If dupli- cate samples
from several sources
are combined, then
the precision of the measure- ment process must be approximately the same for each. The precision obtained
is then compared with the precision needed to accept
the results of the analysis.
The Analysis of Blanks
The
use
of
a blank was introduced
as a means of correcting the measured signal for contributions from sources other than
the analyte. The most common
blank is a method, or reagent blank,
in which an analyte-free sample, usually distilled
water, is carried through the analysis using
the same reagents, glassware, and instrumentation. Method blanks are used to identify and
correct systematic errors
due to impurities in the reagents and con-
tamination in the glassware and instrumentation. At a minimum, method blanks should be analyzed whenever new reagents are
used, although a more frequent analysis provides
an ongoing monitoring
of the purity of the reagents. A new method
blank should also
be run whenever a sample with
a high concentration of the analyte is analyzed, because
any residual carryover of the analyte
may contami- nate the glassware or instrumentation.
When samples are collected in the field, the method blank may be augmented with field and trip
blanks. A field blank is
an analyte-free sample
carried from the laboratory to the sampling
site. At the sampling site the blank
is transferred to a
clean sample container, exposing it to the local environment, preserved, and trans- ported back to the
laboratory for analysis. Field blanks are
used to identify and correct systematic errors due to sampling, transport, and analysis. Trip blanks are analyte-free samples
carried from the laboratory to the sampling
site and returned to the laboratory without
being opened. A trip blank
is used to identify and
correct systematic errors due
to cross-contamination of volatile organic
compounds during transport, handling, storage, and analysis.
Analysis of Standards
The analysis of a
standard containing a known concentra- tion
of analyte also
can be used
to monitor a system’s state
of statistical control. Ide- ally, a standard
reference material (SRM) should be used, provided
that the matrix of
the SRM is similar to that of the samples
being analyzed. A variety of appropriate
SRMs
are available from the National Institute of Standards and Technology
(NIST). If a suitable SRM is not available, then an independently prepared synthetic
sample can be used if it is prepared from
reagents of known
purity. At a minimum,
a standardization of the method is verified
by periodically analyzing
one of the cali- bration standards. In all cases,
the analyte’s experimentally determined concentra-
tion in the standard must fall within
predetermined limits if the system
is to be con- sidered
under statistical control.
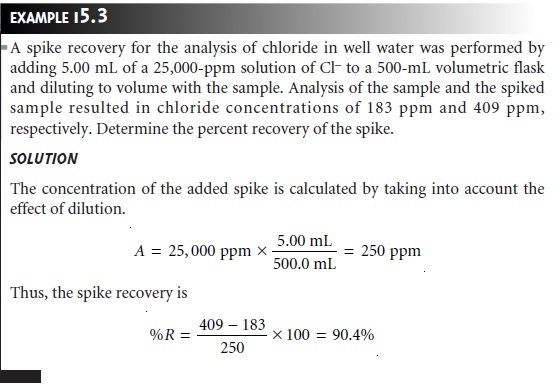
Spike Recoveries
One of the most important quality assessment tools
is the recov- ery of a known addition, or spike, of analyte to a method
blank, field blank,
or sam- ple. To determine a spike recovery, the
blank or sample
is split into
two portions, and a known amount
of a standard solution of the analyte
is added to one portion. The concentration of the analyte is determined for both the spiked, F, and
unspiked portions, I, and the
percent recovery, %R, is
calculated as
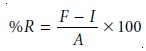
where A is the
concentration of the analyte added to the spiked portion.
Spike recoveries on method blanks
and field blanks
are used to evaluate the general performance of an analytical procedure. The concentration of analyte added to
the blank should
be between 5 and 50 times the
method’s detection limit.
Sys- tematic errors occurring during sampling and transport will result in an unaccept- able recovery for the field blank,
but not for the method
blank. Systematic errors occurring in the laboratory, however, will affect the recoveries for both the field and method blanks.
Spike recoveries for
samples are used
to detect systematic errors due to the
sample matrix or the stability of the sample
after its collection. Ideally, samples
should be spiked in the
field at a concentration between
1 and 10 times the
expected concentration of the analyte or 5 to 50 times the method’s
detection limit, whichever is larger. If the recovery
for a field spike is unacceptable, then a sample
is spiked in the laboratory and analyzed immediately. If the recovery
for the labora- tory spike is acceptable, then the poor
recovery for the
field spike may
be due to the
sample’s deterioration during storage. When the recovery for the laboratory spike also is unacceptable, the
most probable cause
is a matrix-dependent relationship be- tween the analytical signal
and the concentration of the analyte.
In this case the
samples should be analyzed by the method of standard
additions. Typical limits for
acceptable spike recoveries for the analysis
of waters and wastewaters are shown in Table
15.1.
External Methods of Quality Assessment
Internal methods of quality assessment should always be viewed with some level of
skepticism because of the potential for bias in their execution and interpretation. For this
reason, external methods
of quality assessment also play an important role in quality assurance programs. One
external method of quality assessment is the
certification of a laboratory by a sponsoring agency. Certification is based on the
successful analysis of a set
of proficiency standards prepared by the sponsoring agency. For example,
laboratories involved in environmental analyses may be re- quired
to analyze standard
samples prepared by the Environmental Protection
Agency. A second
example of an external method
of quality assessment is the volun- tary participation of the laboratory in a collaborative test sponsored by a professional organization such as the Association of Official Analytical Chemists. Finally, individuals contracting with a laboratory
can perform their own external quality assessment by submitting blind
duplicate samples and blind standard
sam- ples to the laboratory for analysis. If the results
for the quality
assessment samples are unacceptable, then there is good reason
to consider the results suspect
for other samples provided
by the laboratory.
Related Topics