Chapter: Mechanical : Engineering Thermodynamics : Properties of a Pure Substance and Steam Power Cycle
Pure Substance
PURE SUBSTANCE
A pure substance is one
that has homogeneous and invariable chemical composition. It may exist in more
than one phase, but chemical composition is same for all the phases. Thus,
water, mixture of water and ice and water and steam are all examples of pure
substance. Sometimes a mixture of gases e.g. air is considered as pure
substance.
We
have seen that two independent properties are sufficient to determine
thermodynamic state of a fluid when it is in equilibrium. Any other
thermodynamic property is a function of the chosen pair of independent
properties. We shall first consider the relation between the primary properties
p, v and T, the equation expressing this relation for any particular fluid
being called the equation of state or characteristic equation of the fluid.
Since we have three
variables to consider, the obvious procedure is to measure the variation of one
with another while the third is kept constant and repeat this for a range of
values of the third variable.
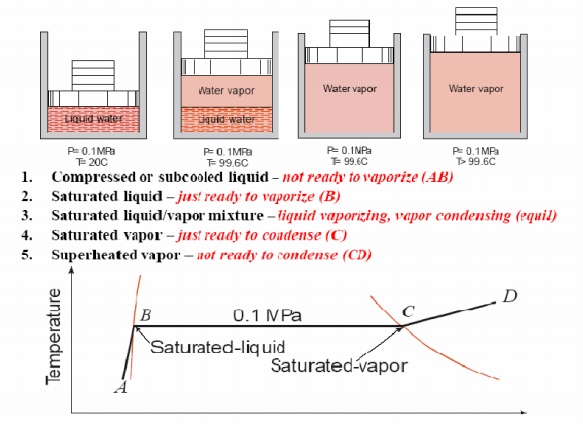
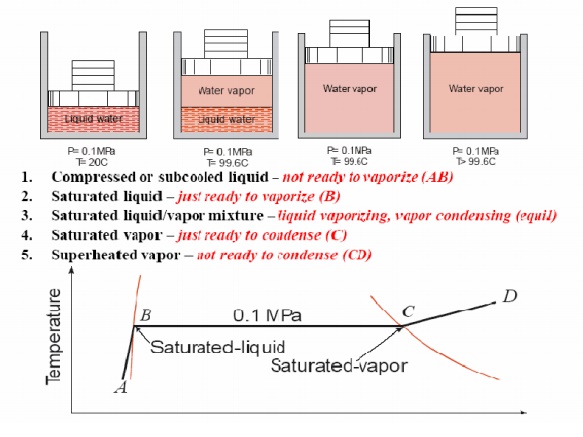
FORMATION OF STEAM AND PROPERTIES
Imagine
unit mass of ice below the freezing point, enclosed in a cylinder by a piston
under a constant load of 1 atmosphere (1 atm. = 1.01325 bar = 101.325 kPa). If
heat is added to the cylinder while keeping the pressure constant, the
temperature rises and ice expands until a temperature of 273.15 K (00C) is
reached (AB) as shown in Fig. Further heating does not raise the temperature of
ice but causes a change to the liquid phase (BC). The change of phase occurs at
a constant temperature and by reduction of specific volume. The heat required
for this process is known as latent heat of fusion. Further
heating results in a rise of temperature of liquid and a further contraction in
volume until the temperature is about 40C and subsequent expansion until a
temperature of 373.15 K (1000C) is reached (point D). At this point a second
phase change occurs at constant temperature with a large increase in volume
until the liquid has been vaporised (point E). The heat required in this case
is called the latent heat of vaporisation. When vaporisation is
complete, the temperature rises again on heating (line EF). The heat
transferred to a substance while the temperature changes is sometimes referred
to as sensible heat. This constant pressure lines
are called isobars.
Related Topics