Chapter: Biochemistry: Proteins
Properties of amino acids
Properties of amino acids
1. Physical properties
Amino acids are coloureless, crystalline,
generally soluble in water, in acid and in alkali but sparingly soluble in
organic solvents.
2. Chemical properties
i. Ionic forms of amino acids
Amino acids bear atleast two ionizable weak
acid groups, a - COOH and a -NH2. In solution, two forms of these
groups, one charged and another uncharged, exist in protonic equilibrium:
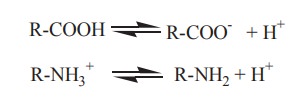
R-COOH and R-NH3+
represent the protonated or acidic partners in these equilibria.
R-COO- and R-NH2 are the conjugate
bases of the corresponding acids.
ii. Zwitterion
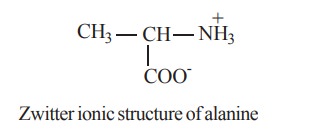
Zwitter ionic structure of alanine
Due to the presence of an acidic and a basic
group in the same molecule, the amino acid exists largely as a dipolar ion or a
zwitterion, which can react as an acid as well as a base. In zwitterion, the
proton from the carboxylic group is transferred to the amino group. Thus a
zwitterion carries both positive and negative charges.
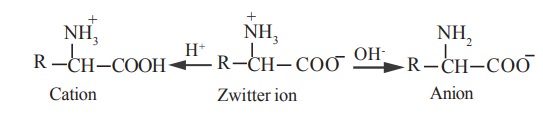
In acidic solution an amino acid behave like a
protonated derivative and therefor migrates to the cathode under electric
field. In an alkaline medium, the same amino acid behaves like an anion
derivative and therefore migrates to the anode.
iii. Isoelectric point
The net charge (the algebraic sum of all the
positively and negatively charged groups present) of an amino acid depends upon
the pH, or proton concentration of the surrounding medium.
The pH at which an amino acid bears no net
charge and hence does not migrate to either of the anode or cathode under the
influence of an electric current, is known as the isoelectric point or
isoelectric pH.
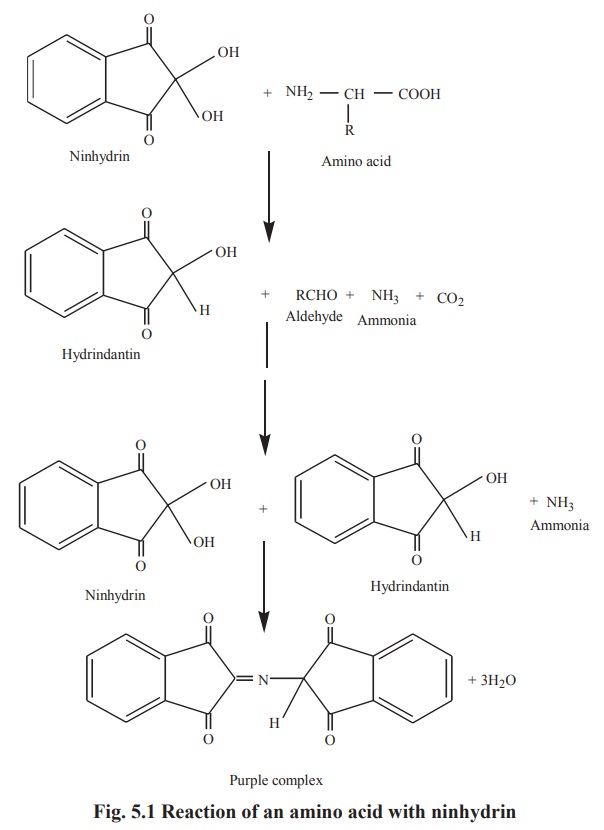
iv. Reaction with ninhydrin
Ninhydrin oxidatively decarboxylates an amino
acid to CO2, NH 3 and an aldehyde. The reduced ninhydrin
then reacts with the liberated ammonia forming a purple complex, which absorbs
light at a wavelength of 570 nm.
Related Topics