Chapter: Environmental Biotechnology: Pollution and Pollution Control
Practical Toxicity Issues
Practical Toxicity Issues
The general factors which influence toxicity have already been set
out earlier in this discussion, but before moving on to consider wider
practical issues it is helpful to mention briefly the manner in which the
toxic action of pollutants arises. There are two main mechanisms, often labelled
ŌĆśdirectŌĆÖ and ŌĆśindirectŌĆÖ. In the former, the effect arises by the contaminant
combining with cellular constituents or enzymes and thus preventing their
proper function. In the latter, the damage is done by secondary action
resulting from their presence, typified by histamine reactions in allergic
responses.
The significance of natural
cycles to the practical applications of environmental biotechnology is a point
that has already been made. In many respects the functional toxicity of a
pollution event is often no more than the obverse aspect of this same coin, in
that it is frequently an overburdening of existing innate systems which
constitutes the problem. Thus the difficulty lies in an inability to deal with
the contaminant by normal routes, rather than the simple presence of the
substance itself. The case of metals is a good example. Under normal
circumstances, processes of weathering, erosion and volcanic activity lead to
their continuous release into the environment and corresponding natural
mechanisms exist to remove them from circulation, at a broadly equivalent rate.
However, human activities, particularly after the advent of industrialisation,
have seriously disrupted these cycles in respect of certain metals, perhaps
most notably cadmium, lead, mercury and silver. While the human contribution
is, clearly, considerable, it is also important to be aware that there are
additional potential avenues of pollution and that other metals, even though
natural fluxes remain their dominant global source, may also give rise to
severe localised contamination at times.
The toxicity of metals is
related to their place in the periodic table, as shown in Table 4.1 and
reflects their affinity for amino and sulphydryl groups (associated with active
sites on enzymes).

In broad terms, type-A
metals are less toxic than type-B, but this is only a generalisation and a
number of other factors exert an influence in real-life situations. Passive
uptake by plants is a two-stage process, beginning with an initial binding onto
the cell wall followed by diffusion into the cell itself, along a concentration
gradient. As a result, those cations which readily associate with particulates
are accumulated more easily than those which do not. In addition, the presence
of chelating ligands may affect the bio-availability and thus, the resultant
toxicity of metals. Whereas some metal-organic complexes (Cu-EDTA for example)
can detoxify certain metals, lipophilic organometallic complexes can increase
uptake and thereby the functional toxic effect observed.
Although we have been
considering the issue of metal toxicity in relation to the contamination of
land or water, it also has relevance elsewhere and may be of particular
importance in other applications of biotechnologies to environmental problems.
For example, anaerobic digestion is a engineered microbial process commonly
employed in the water industry for sewage treatment and gaining acceptance as a
method of biowaste management. The effects of metal cations within anaerobic
bioreactors are summarised in Table 4.2, and from which it is apparent that
concentration is the key factor.
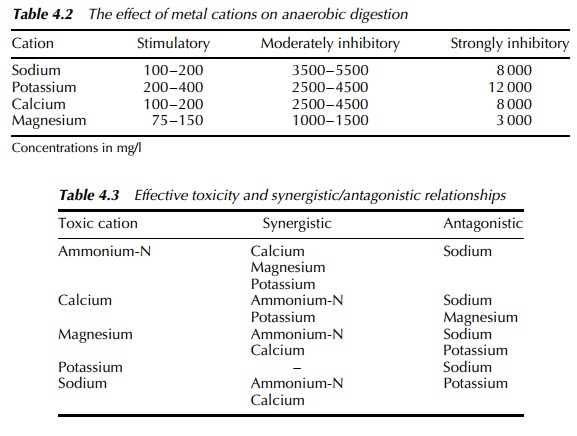
However, the situation is
not entirely clear cut as the interactions between cations under anaerobic
conditions may lead to increased or decreased effective toxicity in line with
the series of synergistic/antagonistic relationships shown in Table 4.3.
Toxicity is often dependent
on the form in which the substance occurs and substances forming analogues
which closely mimic the properties of essential chemicals are typically readily
taken up and/or accumulated. Such chemicals are often particularly toxic as the
example of selenium illustrates.
Often wrongly referred to as
a toxic metal, and though it has some metallic properties, selenium is a
nonmetal of the sulphur group. It is an essential trace element and naturally
occurs in soils, though in excess it can be a systemic poison with the LD50 for
certain selenium compounds being as low as 4 micrograms per kg body weight.
In plants, sulphur is actively taken up in the form of sulphate
SO42ŌłÆ. The similarity of selenium to sulphur leads to the existence of similar
forms in nature, namely selenite, SeO32ŌłÆ and selenate SeO42ŌłÆ.
As a result, selenium can be taken up in place of sulphur and
become incor-porated in normally sulphur-containing metabolites.
Related Topics