Chapter: Biochemistry: Protein Synthesis: Translation of the Genetic Message
Posttranslational Modification of Proteins
Posttranslational Modification of
Proteins
Newly
synthesized polypeptides are frequently processed before they reach the form in
which they have biological activity. We have already mentioned that, in
prokaryotes, N-formylmethionine is
cleaved off. Specific bonds in precursors can be hydrolyzed, as in the cleavage
of preproinsulin to proinsulin and of proinsulin to insulin (Figure 12.22).
Proteins destined for export to specific parts of the cell or from the cell
have leader sequences at their N-terminal ends. These leader sequences, which
direct the proteins to their proper destination, are recognized and removed by
specific proteases associated with the endoplasmicreticulum.
The finished protein then enters the
Golgi apparatus, which directs itto its final destination.
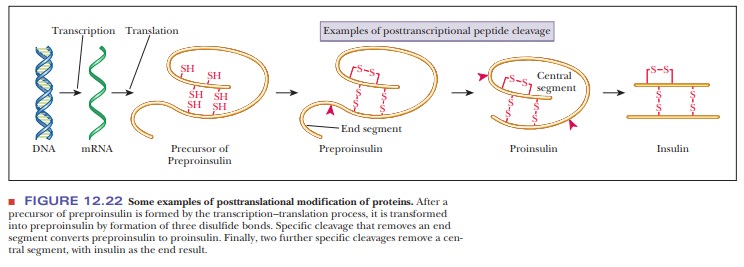
In addition to the processing of proteins by breaking bonds, other sub-stances can be linked to the newly formed polypeptide. Various cofactors, such as heme groups, are added, and disulfide bonds are formed (Figure 12.22). Some amino acid residues are also covalently modified, as in the conversion of proline to hydroxyproline. Other covalent modifications can take place, an example being the addition of carbohydrates or lipids to yield an active final form of the protein in question. Proteins can also be methylated, phosphory-lated, and ubiquitinylated.
Once modified, do proteins always have the correct three-dimensional structure?
A highly
important question concerns the proper folding of the newly synthesized
protein. In principle, the primary structure of the protein conveys enough
information to specify its three-dimensional structure. In the cell, the
complexity of the process and the number of possible conformations make it less
likely that a protein would spontaneously fold into the correct conformation.
Summary
Proteins are usually modified after their initial translation.
Protein modification includes removal of the N-formylmethionine from prokaryotic
proteins, cleavage of specific amino acids, and addition of signal sequences.
Nonprotein components can be added to some proteins, such as the
heme group added to hemoglobin.
Proteins
must also be folded properly into their correct form. Protein molecules called
chaperones help many proteins fold into their correct form.
Related Topics