Chapter: Biochemistry: Protein Synthesis: Translation of the Genetic Message
Amino Acid Activation
Amino Acid Activation
The
activation of the amino acid and the formation of the aminoacyl-tRNA take place
in two separate steps, both of which are catalyzed by the aminoacyl-tRNA
synthetase (Figure 12.6). First, the amino acid forms a covalent bond to an
adenine nucleotide, producing an aminoacyl-AMP. The free energy of hydrolysis
of ATP provides energy for bond formation. The aminoacyl moiety is then transferred
to tRNA, forming an aminoacyl-tRNA.

Aminoacyl-AMP
is a mixed anhydride of a carboxylic acid and a phosphoric acid. Because
anhydrides are reactive compounds, the free-energy change for the hydrolysis of
aminoacyl-AMP favors the second step of the overall reaction. Another point
that favors the process is the energy released when pyrophosphate (PPi) is
hydrolyzed to orthophosphate (Pi) to replenish the phosphate
pool in the cell.
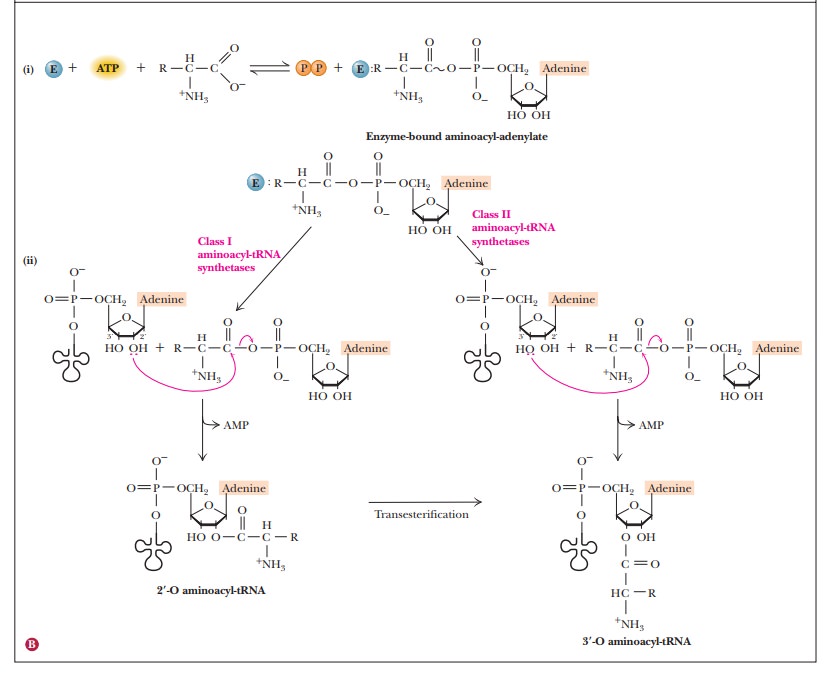

In the
second part of the reaction, an ester linkage is formed between the amino acid
and either the 3'-hydroxyl or the 2'-hydroxyl of the ribose at the 3' end of
the tRNA. There are two classes of aminoacyl-tRNA synthetases. Class I loads
the amino acid onto the 2' hydroxyl. Class II uses the 3' hydroxyl. These two
classes of enzyme appear to be unrelated and indicate a convergent evolu-tion.
Several tRNAs can exist for each amino acid, but a given tRNA does not bond to
more than one amino acid. The synthetase enzyme requires Mg2+ and is
highly specific both for the amino acid and for the tRNA. A separate synthetase
exists for each amino acid, and this synthetase functions for all the different
tRNA molecules for that amino acid. The specificity of the enzyme contributes
to the accuracy of the translation process. The synthe-tase assures that the
right amino acid pairs up with the right tRNA, and this is its primary
function. The synthetase has another level of activity as well. An extra level
of proofreading by the synthetase is part of what is sometimes called the
ŌĆ£second genetic code.ŌĆØ
What is the ŌĆ£second genetic codeŌĆØ?
The
two-stage reaction allows for selectivity to operate at two levels: that of the
amino acid and that of the tRNA. The specificity of the first stage uses the
fact that the aminoacyl-AMP remains bound to the enzyme. For example,
isoleucyl-tRNA synthetase can form an aminoacyl-AMP of isoleucine or the
structurally similar valine. If the valyl moiety is then transferred to the
tRNA for isoleucine, it is detected by an editing site in the tRNA synthetase,
which then hydrolyzes the incorrectly acylated aminoacyl-tRNA. The selectivity
resides in the tRNA, not in the amino acid.
The second aspect of selectivity depends on the selective recognition of tRNAs by aminoacyl-tRNA synthetases. Specific binding sites on tRNA are rec-ognized by aminoacyl-tRNA synthetases. The exact position of the recognition site varies with different synthetases, and this feature, in and of itself, is a source of greater specificity. Contrary to what one might expect, the anticodon is not always the part of the tRNA that is recognized by the aminoacyl-tRNA synthe-tase, although it frequently is involved. Figure 12.7 shows the locations of the recognition sites for the tRNAs for various amino acids.
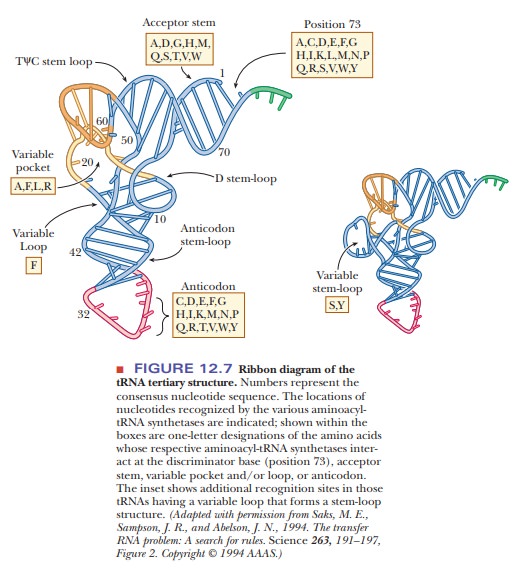
The
recognition of the correct tRNA by the synthetase is vital to the fidelity of
translation because most of the final proofreading occurs at this step.
Summary
Before amino acids can be incorporated into a
peptide, they must be activated.
Amino acids are activated
through a reaction with ATP yielding an amino acid bonded to AMP. The AMP-bound
amino acid is then reacted with a tRNA to yield an amino acyl tRNA.
The
enzymes responsible for activation are called aminoacyl-tRNA synthetases.
Related Topics