Chapter: Biochemistry: Protein Synthesis: Translation of the Genetic Message
Chaperones: Preventing Unsuitable Associations
Chaperones: Preventing Unsuitable
Associations
It is sometimes said that a chaperone’s task is to prevent unsuitable associations. The class of proteins known as molecular chaperones operate in this way by preventing aggregation of newly formed proteins until they fold into their active forms. The information necessary for protein folding is present in the amino acid sequence, and many proteins fold correctly without any outside help, as shown in part (a) of the figure. However, some proteins may form aggregates with other proteins, or may fold with incorrect secondary and tertiary structures, unless they interact first with a chaperone. Well-known examples include heat-shock proteins, which are produced by cells as a result of heat stress. The prime examples of this are the Hsp70 class of proteins, named after the 70-kDa heat-shock protein that occurs in mammalian cytosol, shown in part (b) of the figure. The Hsp70 protein binds to the nascent polypeptide and prevents it from interacting with other proteins or from folding into an unproductive form. Completion of correct folding requires release from the chaperone and is driven by ATP hydrolysis. All proteins in this class, which were first studied as a response to heat stress in cells of all types, have highly conserved primary structures, in both prokaryotes and eukaryotes.
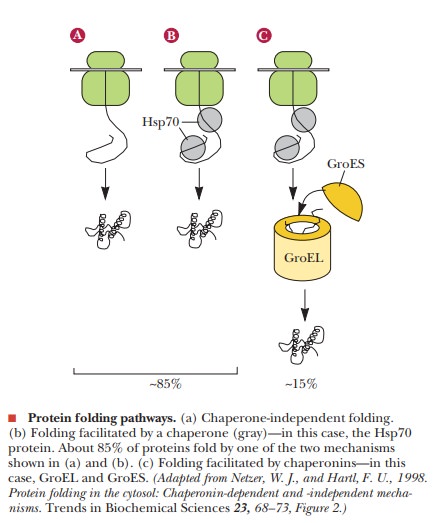
About 85% of proteins fold as shown in parts (a) and of the
figure. Another group, the chaperonins
(also called the Hsp60 proteins from
their 60-kDa molecular weight) are knownto be involved in the folding of the
other 15% of proteins. A large multisubunit protein forms a cage of 60-kDa
subunits around the nascent protein to protect it during the folding process,
shown in part (c) of the figure. GroEL
and GroES are the best-characterized
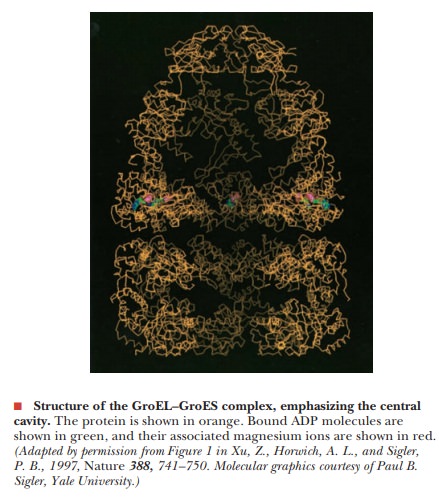
chaperonins from E. coli. GroEL is
formed by two stacked seven-membered rings of 60-kDa subunits with a central
cavity, as shown in the figure below. Protein folding occurs in the central
cavity and is dependent on ATP hydrolysis. GroES is a single seven-membered
ring of 10-kDa subunits that sits on top of GroEL. During protein folding, the
polypeptide chain goes through cycles of binding and unbinding to the surface
of the central cavity. In some cases, more than 100 ATP molecules must be
hydrolyzed before protein folding is complete.
Related Topics