Chapter: The Diversity of Fishes: Biology, Evolution, and Ecology: Conservation and the future of fishes
Pollution - General causes of fish biodiversity decline
Pollution
Pollution enters aquatic systems as sediments or in the form of dissolved or suspended substances in runoff or precipitation, while attached to sediments, or while airborne. Humanproduced toxic substances number in the thousands, ranging from elemental contaminants such as chlorine and heavy metals to chemical complexes such as persistent pesticides, detergents, endocrine disrupting compounds (EDCs), and petroleum products (NRC 1999a). Harmful effects on fishes occur as a result of direct toxicity, by interfering with developmental pathways in the case of EDCs, or through food chain effects (e.g., eutrophication, bioaccumulation), ultimately affecting individual survival and reproduction. Food chain effects also link contaminated fishes to other endangered species such as marine and terrestrial mammals and birds of prey (Lloyd 1992; Ewald et al. 1998).
Pollution-related reductions in fish biodiversity occur worldwide. Some of the best documented examples have occurred in North America and Europe due to acid rain and agricultural chemicals. Acid rain has a pH of less than 5.6. It results when oxides of nitrogen and sulfur (NOx, SO4) from internal combustion engines and coal-burning operations are further oxidized in the atmosphere to form nitric and sulfuric acid. Acid rain becomes a particularly serious problem in watersheds composed of rock types that are incapable of buffering the acids, such as the metamorphic rocks of northern North America and Europe. Most acid rain-contaminated systems suffer prolonged periods of low pH, but episodic inputs during snowmelt or storms can exacerbate already stressful conditions, including increased acute toxicity from aluminum and mercury (see Gensemer & Playle 1999). Mercury mobilization occurs because bacteria convert mercury to methylmercury more rapidly at lower pH. Spring rainstorms and snowmelt are especially injurious: acidic compounds accumulate in winter snowpack, flushing occurs when eggs and larvae are most abundant, and early life stages are particularly vulnerable to low pH (e.g., Sullivan 2000).
Acid rain has caused dramatic chemical changes in more than 100,000 lakes in Ontario and Quebec, wiping out all wild stocks of the endangered Aurora Trout (Salvelinus fontinalis timagiensis), and has reduced the range of the endangered Acadian Whitefish (Coregonus huntsmani) by 50% (Williams et al. 1989). Similar acidifi cation and salmonid declines have occurred in the Adirondack Mountains of New York and in many Scandinavian lakes. Acid deposition is considered a prime contributor to the decline of Atlantic Salmon stocks in eastern Canada and will probably prevent their recovery (e.g., Watt et al. 2000). Fish kills in Norway following episodic acidifi cation affected both Atlantic Salmon and Brown Trout (Baker & Christensen 1991). Norway lost 18 stocks of Atlantic Salmon, with eight more considered threatened, and Brown Trout have disappeared from 39% of Norway’s lakes, with significant declines in another 17% (Sandøy & Langåker 2001). Brook Trout, which are relatively acid tolerant, have disappeared from approximately 11% of the lakes in the Adirondack Mountains of New York due to acidifi cation (Baker et al. 1993). Minnows are even more acid sensitive and have disappeared from 19% of surveyed lakes.
Agricultural chemicals – pesticides, herbicides, and fertilizers – have been responsible for the extermination of many fishes in the American southwest, particularly those in isolated habitats. The toxic chemicals work directly on the fishes or are ingested with food, whereas fertilizers lead to eutrophication, which changes the balance of algae from edible species to inedible blue-greens, raises lake temperature, and lowers oxygen content. The Clear Lake Splittail, Pogonichthys ciscoides, a cyprinid endemic to Clear Lake in northern California, was extremely abundant through the 1940s. Agricultural development of the lake basin transformed the lake from a clear, cool habitat dominated by native fishes to a warm, turbid lake dominated by introduced species. The last Splittail was taken from the lake in 1970. Eutrophication or toxic chemicals have been similarly implicated in the demise of such unusual fishes as the Lake Ontario Kiyi, Phantom Shiner, Stumptooth Minnow, Blue Pike, and Utah Lake Sculpin. Overall, pollution has contributed to the demise of 15 of the 40 species and subspecies of fishes that have gone extinct in North America during the past century (Williams et al. 1989).
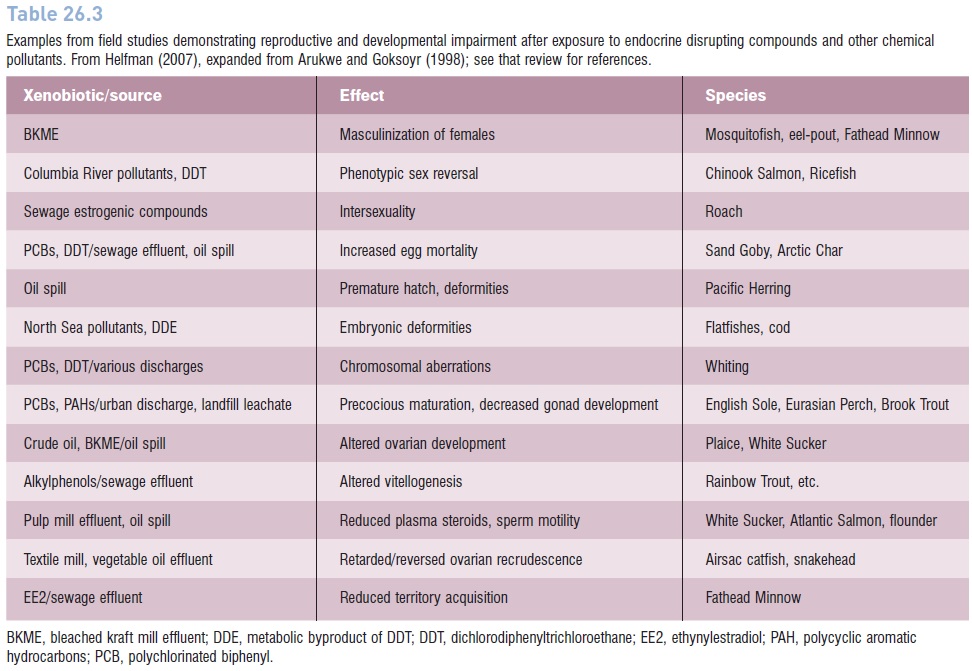
Endocrine disrupters are an insidious form of pollution because they interfere with growth and development at extremely low chemical concentrations, with consequences that we are only now coming to appreciate (Colborn et al. 1996; Arcand-Hoy & Benson 1998; NRC 1999a). In fishes, EDCs affect sexual differentiation and reproductive performance, acting early in sex determination as well as later when gonads produce sex products (Devlin & Nagahama 2002). Documented impacts on wild populations are reported increasingly and include abnormal gonad morphology, reduced rates of sperm and egg production and release, and reduced quality of gametes (Arukwe 2001) (Table 26.3). Other effects are altered reproductive behavior and unnatural sex reversal or failure to mature, with reproductive failure an ultimate result (e.g., Jones & Reynolds 1997).
Table 26.3
Examples from field studies demonstrating reproductive and developmental impairment after exposure to endocrine disrupting compounds and other chemical pollutants. From Helfman (2007), expanded from Arukwe and Goksoyr (1998); see that review for references.
EDCs contribute to and exacerbate declines among imperiled fishes. The Columbia River of Oregon and Washington, previously the most productive salmon river in America, suffers from damming, overfishing, introductions from fish hatcheries, and agricultural and industrial pollution. Columbia River water now contains at least 92 chemical contaminants found in fish samples, including 14 metals, DDT, chlordane, polychlorinated biphenyls (PCBs), and chlorinated dioxin and furans (USEPA 2002); some of these chemicals are known endocrine disrupters. Approximately 85% of female-appearing Chinook Salmon sampled from the Columbia River possessed a genetic marker for the Y chromosome, indicating that they were in fact sexreversed males (Nagler et al. 2001). When XY females mate with normal XY males, 25% of the F1 generation can be expected to exist as YY males, skewing the population sex ratio from a normal 1 : 1 to a male dominated 3 : 1. Subsequent matings could increase the proportion of males as YY males mated with normal XX females, which would be potentially disastrous for already stressed populations.
Fishes as indicators of environmental health
“The quality of fishing reflects the quality of living.” This motto of the American Sportfishing Association, although focusing on exploitable species, summarizes the host of problems facing aquatic ecosystems worldwide. Lakes, rivers, and oceans with abundant, diverse fishes are reliable indicators of a healthy environment for all life forms. Quantifying the condition of aquatic habitats therefore becomes a crucial exercise in understanding and predicting potential hazards to human welfare. Fishes can serve asindicators of the health of aquatic systems, in advance of effects on human health. At one extreme, massive fish kills indicate high levels of lethal contaminants, or low levels of oxygen. Ideally, less acute warnings are preferable. To this end, several measures have been developed that use quantifiable aspects of fish assemblage structure, health, and behavior as a means of monitoring conditions in aquatic systems. One approach used widely is the index of biotic integrity or IBI (Karr 1981, 1991; Miller et al. 1988a; Karr & Chu 1999), which combines measurements of species composition, abundance, and trophic relationships for different habitats. An IBI provides a quantitative comparison between the habitat in question and “unimpaired” reference systems to assess relative degrees of disturbance. The IBI bases its comparisons on a number of traits that generally characterize disturbed systems, such as an increase in number of introduced species, replacement of specialist species with generalist species, decline in the number of sensitive species, impair ment of reproduction, change in age structure of populations away from older age classes, and an increase in disease and anatomical anomalies. The IBI was originally developed for midwestern US streams, but has been applied successfully in a variety of systems (Hughes & Noss 1992; Simon & Lyons 1995).
Environmental contamination is more conventionally investigated by assaying water and sediments for known toxins, correlating growth abnormalities with sediment contaminants, or by observing the responses of fishes exposed to suspect water (Heath 1987; Gassman et al. 1994). Traditionally, the concentration at which 50% of the animals die (LD50) is considered a critical threshold. Lower levels of contamination can be indicated by behavioral measures, such as elevated breathing rates, coughing, chafing against the bottom, impaired locomotion and schooling, and suppressed activity or hyperactivity. Although relatively rapid, such bioassays primarily measure immediate conditions. The measurement of “body burdens” of bioaccumulated contaminants in fish tissues gives a broader picture, but can vary with season, feeding habits, or metabolic activity.
A more integrated, long-term picture can be obtained by measuring alterations in energetics, metabolism, growth, reproduction, and behavior (the biomarker approach of Hellawell (1983) and McCarthy and Shugart (1990)). At a biochemical and energetic level, stress is indicated by changes in such attributes as liver enzyme function, occurrence of DNA damage, unusual ratios of intermediate metabolites (ADP : ATP), amounts of or ability to store lipids, and growth and developmental anomalies. Histological markers include parasite loads and damage, tissue necrosis or abnormal growth (particularly pathologies of the gills and liver), and both elevation and suppression of immune responses. At the population level, reproductive output can be monitored, whereas species richness, presence/ absence of sensitive species, and indices such as the IBI indicate assemblage and community-level effects. These measures are useful for monitoring water quality as it directly affects fishes, but also because fishes are effective sentinels against human health problems (Adams 1990; McCarthy & Shugart 1990). Many of the responses listed in Table 26.3 can be considered biomarkers.
Related Topics