Chapter: Medical Microbiology: An Introduction to Infectious Diseases: Plague and Other Bacterial Zoonotic Diseases
Plague
PLAGUE
Plague, an infection of rodents transmitted to humans by the bite of infected fleas, is the most explosively virulent disease known. Most cases begin with a painful swollen lymph node (bubo) from which the bacteria rapidly spread to the blood-stream. Plague pneumonia (Black Death) is produced by seeding from the blood-stream or from another patient with pneumonia. All forms cause a toxic picture with shock and death within a few days. No other disease regularly kills previ-ously healthy persons so rapidly.
EPIDEMIOLOGY
The term plague is often used generically to describe any explosive pandemic disease with high mortality. Medically, it refers only to infection caused by Y. pestis, and this ap-plication was justly earned, because Y. pestis was the cause of the most virulent epidemic plague of recorded human history, the Black Death of the Middle Ages. In the 14th cen-tury, the estimated population of Europe was 105 million; between 1346 and 1350, 25 million died of plague. Pandemics continued through the end of the 19th century and the early 20th century despite elaborate quarantine measures developed in response to the obvious communicability of the disease. Yersin isolated the etiologic agent in China in 1894 and named it after his mentor, Pasteur (Pasteurella pestis). The name was later changed to honor Yersin (Yersinia pestis).
Plague is a disease of rodents transmitted by the bite of rat fleas (Xenopsylla cheopis) that colonize them. It exists in two interrelated epidemiologic cycles, the sylvatic and the urban (Fig 32–1). Endemic transmission among wild rodents in the sylvatic (L. sylvaticus, belong-ing to or found in the woods) is the primary reservoir of plague. When infected rodents enter a city, circumstances for the urban cycle are created. Humans can enter the cycle from the bite of the flea in either environment. However, chances are greater in the urban setting.
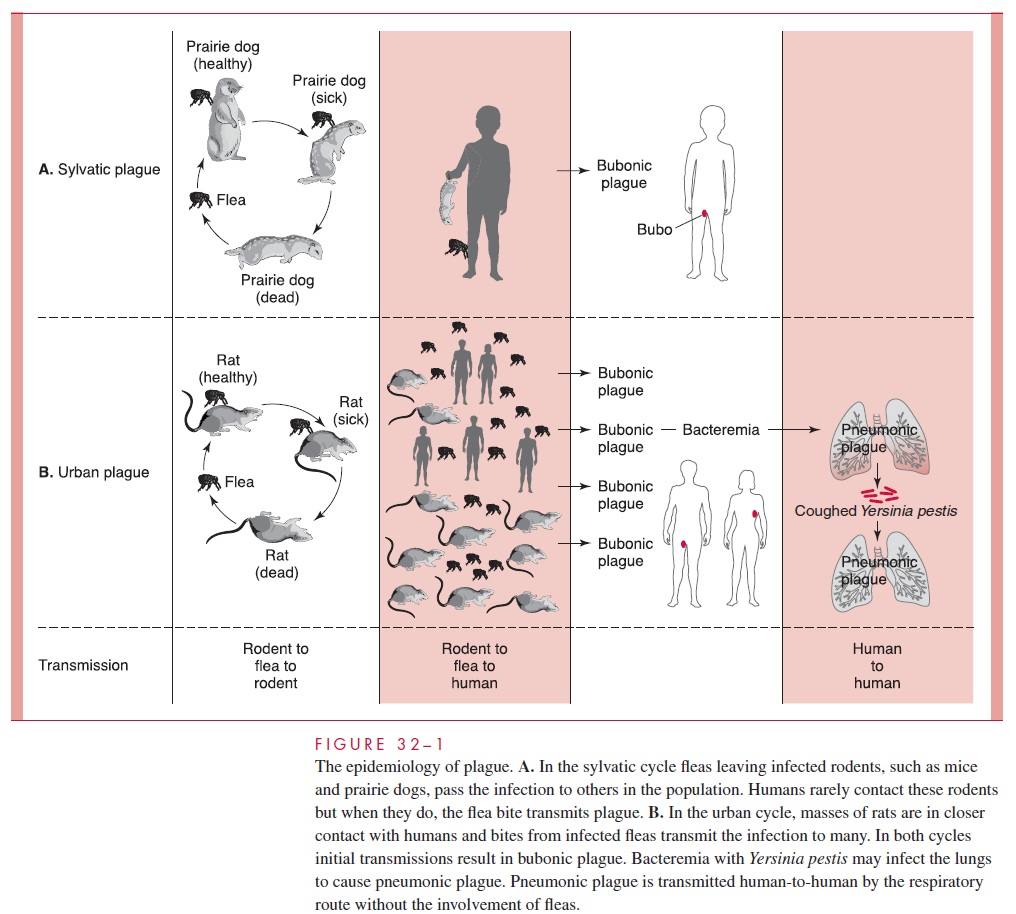
The plagues of the Middle Ages are examples of the urban cycle involving rats and humans. When food is scarce in the countryside, rats migrate to cities, which facilitates rat-to-rat transmission and brings the primary reservoir into closer contact with humans. When the number of nonimmune rats is sufficient, epizootic plague develops among them, with bacteremia and high mortality. Fleas feeding on the rats become infected, and the bacteria multiply in the intestinal tract of the fleas to numbers that eventually block the proventriculus. As an infected rat dies, its hungry fleas seek a new host, which is usually another rat but may be a human. The infected flea regurgitates Y. pestis from the proven-triculus into the new bite wound. Therefore, the probability of transmission to humans is greatest when both rat population and rat mortality are high.
The bite of the flea is the first event in the development of a case of bubonic plague, which, even if serious enough to kill the patient, is not normally contagious to other humans. However, some patients with bubonic plague develop a secondary pneumonia by bacteremic spread to the lungs. This pneumonic plague is highly contagious person-to-person by the respiratory droplet route. It is not difficult to understand how rapid spread proceeds in conjunction with crowded unsanitary conditions and continued flea-to-human transmission. An urban plague epidemic is vividly described through the eyes of a physician in Albert Camus’ novel The Plague.
Although urban plague epidemics have been essentially eliminated by rat control and other public health measures, sylvatic transmission cycles persist in many parts of the world, including North America. These cycles involve nonurban mammals such as prairie dogs, deer mice, rabbits, and wood rats.
Transmission between them involves fleas. Coy-otes or wolves may be infected by the same fleas or by ingestion of infected rodents. By their nature, the reservoir animals rarely come in contact with humans; when they do, however, the infected fleas they carry can transmit Y. pestis. The most common circum-stance is a child exploring the outdoors who comes across a dead or dying prairie dog and pokes, carries, or touches it long enough to be bitten by the fleas leaving the animal. The result is a sporadic case of bubonic plague, which occasionally becomes pneumonic.
Sylvatic plague, which exists in most continents, is common in Southeast Asia but is not found in Western Europe or Australia. In the United States, the primary enzootic areas are the semiarid plains of the western states. Infected animals and fleas have been de-tected from the Mexican border to the eastern half of Washington State. The geographic focus of human plague in the United States is in the “four corners” area where Arizona, New Mexico, Colorado, and Utah meet, but cases have occurred in California, west Texas, Idaho, and Montana. Most years, as many as 15 cases are reported, although this number rose to 30 to 40 in the mid-1980s. These variations are strongly related to changes in the size of the sylvatic reservoir. A fatal case of pneumonic plague reported in 1992 was linked to an infected domestic cat the patient had removed from the crawl space under a rural cabin in the endemic area.
PATHOGENESIS
The plague cycle begins when a rat flea feeds on a rodent infected with Y. pestis. Bacteria are taken with the blood meal and multiply in the infected flea. Some virulence factors such as the fibrinolysin and phospholipase are produced at the ambient temperature (20–28°C), where they may enhance the multiplication of Y. pestis in the flea and facili-tate the agglutination that blocks the flea gut proventriculus. The flea, sensing starvation, feeds voraciously and eventually regurgitates blood and bacteria into the bite wound. If this wound is in a new uninfected host (rat or human), a new case is created.
Once injected past the skin barrier by the flea, Y. pestis produces a new set of viru-lence factors as it senses the change from the temperature and ionic environment of the flea to that of the new host. These include the Yops and an array of other virulence factors, plus the F1 capsular protein and a plasminogen-activating pro-tease. The F1 protein forms a gel-like capsule, which has antiphagocytic properties that allow the bacteria to persist and multiply in the submucosa. The organisms eventually reach the regional lymph nodes through the lymphatics, where they multiply rapidly and produce a hemorrhagic suppurative lymphadenitis known clinically as the bubo. Spread to the bloodstream quickly follows. The extreme systemic toxicity that develops with bac-teremia appears to be due to lipopolysaccharide (LPS) endotoxin combined with the many actions of Yops, proteases, and other extracellular products. The bacteremia causes seeding of other organs, most notably the lungs, producing a necrotizing hemorrhagic pneumonia known as pneumonic plague.
IMMUNITY
Recovery from bubonic plague appears to confer lasting immunity, but for obvious rea- sons the mechanisms in humans have not been extensively studied by modern immuno- logic methods. Animal studies suggest that antibody against the F1 capsular protein is protective by enhancing phagocytosis, but cell-mediated mechanisms are required for in- tracellular killing.
Related Topics