Chapter: Modern Medical Toxicology: Chemical Poisons: Non-Metallic Chemical Poisons
Phosphorus - Chemical Poisons
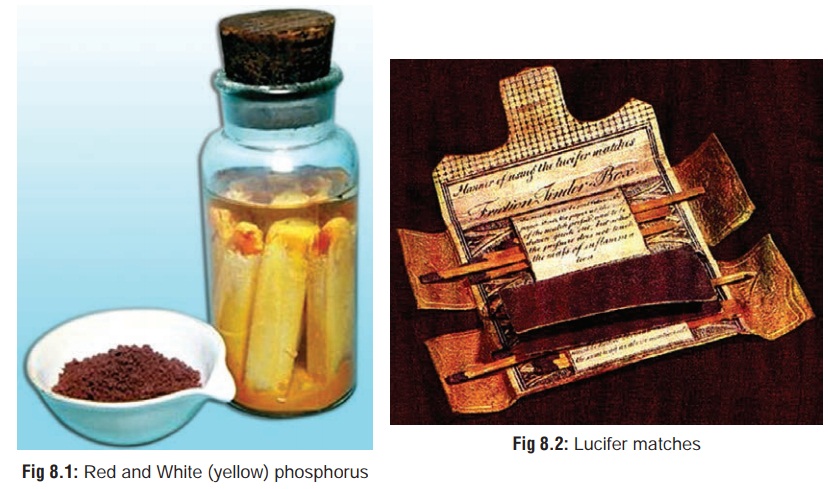
Phosphorus
Physical Appearance
·
The name “phosphorus” is derived from Greek, meaning
“light-bearing”.
·
There are two main varieties (Fig 8.1):

1. Yellow (or White) Phosphorus—
·
This is a yellowish, waxy, crystalline solid with a garlicky
·
odour. On exposure to air, it oxidises into whitish fumes of
phosphorus pentoxide. Hence, it is generally stored under water. Yellow
phosphorus is highly combustible and ignites into flame at 340C. It
is luminescent and glows in the dark (phosphorescence).
2. Red Phosphorus—
·
This is a reddish or brownish, amorphous, odourless
·
substance. It is insoluble and relatively harmless, since it
is not absorbed from the GI tract.
·
“Black phosphorus” is the inert, nontoxic allotropic form of
elemental phosphorus.
·
Derivatives and related compounds of phosphorus include
phosphoric acid, phosphine, aluminium phosphide and zinc phosphide.
Uses
·
Matches:
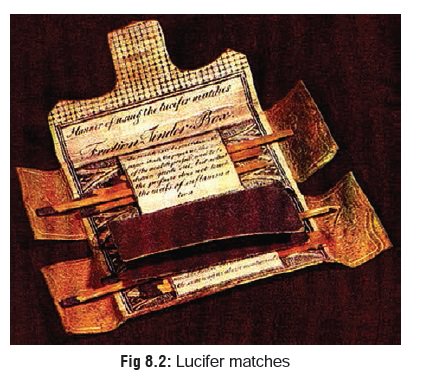
Yellow phosphorus was extensively used in themanufacture of
friction matches during the 19th century. However, because of its propensity to
produce chronic poisoning in workers of the match industry, most countries
agreed at an international convention in Berne, Switzerland in 1906 to prohibit
the manufacture and import of yellow phosphorus for the making of matches.
Hence, these so-called “lucifer matches” gradually (and fortunately) faded out
(Fig 8.2). Today’s “safety match”
contains only potassium chlorate and antimony sulfide (Fig 8.3). It has to be struck against a prepared surface to ignite
it, which is provided by the sides of the match box being coated with powdered
glass and red phosphorus.

·
Fireworks:
Although the use of yellow phosphorus infireworks is
prohibited in Western countries, it is still an important ingredient in several
types of fireworks manu-factured in India.
·
Military
uses: Yellow phosphorus is an ingredient of tracerbullets,
incendiary bombs, smoke screens, and air-sea rescue flares.
·
Insecticide
and rodenticide: There are several pastes andpowders
available in India which contain phosphorus (or zinc phosphide) used for
killing cockroaches and rats. Such pastes are usually mixed with molasses or
butter and spread on bread as bait. Obviously, unintentional ingestion by
chil-dren is quite possible leading to serious poisoning.
Fertiliser
Usual Fatal Dose
About
60 mg (roughly 1 mg/kg body weight).
Mode of Action
·
Yellow phosphorus, a protoplasmic
poison is a potent hepatotoxin (Table
8.1).
·
In large doses, it can cause shock
and cardiovascular collapse since it is also toxic to the heart.
·
Locally, it produces severe
irritation of skin and mucosa.![]()
·
Rate of absorption is greatly
enhanced if phosphorus is administered in an oily vehicle.
Clinical Features
Fulminant Poisoning:
This results from ingestion of a
massive dose, i.e. more than 1 to 2 grams. The dominant clinical picture is one
of peripheral vascular collapse. Death usually occurs in 12 to 24 hours, and
signs of hepatic or renal damage are not seen.
Acute Poisoning:
Today, most cases of phosphorus poisoning fall in this
category. The clinical manifestations characteristically occur in three stages.
a.
First Stage (upto 3 days)—
·
Local effects include severe burning
pain, vomiting, diarrhoea, and abdominal pain.
·
Breath smells of garlic.
·
Vomitus and stools may be luminous
in the dark. There may be haematemesis. Faint fumes may emanate from the stools
(Smoky stool syndrome).
b.
Second Stage (upto several days after the first stagesubsides)—
·
This is an essentially symptom-free
(treacherous) period, and the patient may feel well enough to be discharged
from the hospital.
c,Third
Stage—
·
This is due to the systemic effects
of phosphorus after it has been absorbed.
·
There is a return of the digestive
symptoms with increased severity.
·
In addition, manifestations of liver
damage are prominent—tender hepatomegaly, jaundice which may progress to an
olive green hue, pruritis, bleeding from multiple sites, and finally hepatic
encephalopathy characterised by drowsiness, confusion, ataxia, flapping tremor
of hands (asterixis), stupor, and
coma. At this stage there is a mousy odour to the breath (foetor hepaticus).
·
Renal damage results in oliguria,
haematuria, albu-minuria, and acute renal failure.
·
![]() ECG changes include tachycardia, ST
and T waves changes, QTc prolongation, low voltage QRS, and various
arrhythmias.
ECG changes include tachycardia, ST
and T waves changes, QTc prolongation, low voltage QRS, and various
arrhythmias.
·
There may be terminal convulsions
before death supervenes.
·
Early hypoglycaemia has a grave
prognosis. Survival for three or more days is a good prognostic sign.
Recent reports on phosphorus poisoning have indicated that
the classical three phases of toxicity are not always encountered in these
patients. The incidence of phosphorescent vomitus or faeces, oral mucosal
burns, and presence of a garlicky odour on the breath or in gastric contents
are also quite rare. Therefore, the absence of these findings does not preclude
serious toxicity.
Diagnosis of Acute Poisoning
·
Garlicky odour of breath and
vomitus.
·
Fuming or luminous vomitus and
stools.
·
Evidence of hepatic and renal
failure.
·
Hypokalaemia, hyperchloraemia,
hypocalcaemia and both hyperphosphataemia and hypophosphataemia have been
reported.
·
Hypoprothrombinaemia and
thrombocytopenia may occur following ingestion, and lead to a delayed onset of
haemate- mesis, haematochezia, haematuria, and haemorrhages into the skin and
mucous membranes.
Dermal contact with
phosphorus results in acutely painful corrosion with yellow, necrotic, severely
painful second or third degree chemical burns emitting garlic-like odour.
Absorption from damaged skin may result in acute systemic phosphorus poisoning.
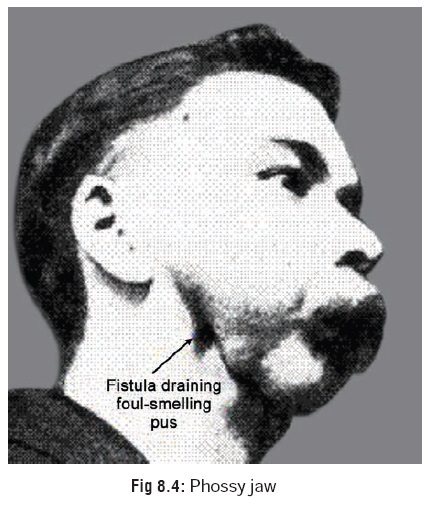
Chronic Poisoning:
This usually results from long-term
occupational exposure to the fumes of phosphorus pentoxide and results in the
condition called Phossy Jaw (Glass Jaw, Lucifer’s Jaw) (Fig 8.4) which was first described by Bristowe in 1862.Main features include toothache (usually origi-nating in a
carious tooth), which because of its recurrence would be eventually extracted
leading to exposure of bone followed by necrosis, sequestra-tion, and
osteomyelitis of jaw (invariably the lower jaw).
Chronic exposure to red phosphorus
or phosphorus sesquisulfide may cause dermatitis.
Treatment
Acute Poisoning:
·
Gastric lavage with potassium
permanganate (1:5000), which oxidises phosphorus into relatively less toxic
phosphoric acid and phosphates. Some authorities recommend administration of
copper sulfate solution (250 gm in a glass of water), which converts phosphorus
to non-toxic copper phosphide. Alternatively, a 0.2% solution of copper sulfate
may be used for stomach wash. It must however be noted that copper sulfate
being a highly toxic substance by itself is not a desir-able antidote, and in
fact is placed in the international list of obsolete antidotes.
·
Do not administer milk or any
oily/fatty foods, since this will enhance the absorption of phosphorus.
·
Vitamin K by IV drip (65 mg) slowly,
to combat hypo-prothrombinaemia.
Intravenous fluids—
·
Isotonic saline and sodium lactate
to treat shock, dehydration, and acidosis.
·
Glucose to combat hypoglycaemia.
·
Calcium gluconate for hypocalcaemia.
·
Whole blood/fresh frozen plasma to
correct coagulation defects.
·
Steroids and inotropic support for
shock.
·
Anticonvulsants for seizures.
Some
investigators suggest the use of N-acetylcysteine (NAC) in patients with stage
I phosphorus toxicity. A dose regimen of
150 mg/kg in 200 cc D5W for 15 minutes, followed by 50 mg/kg in 500 cc D5W for
4 hours, and then 100 mg/kg in 1000 cc D5W for 16 hours is recommended. It is
presumed that NAC may be effective in preventing progression of liver damage
when given in stage I of the illness.
Treatment of dermal burns:
·
After initial flushing with large
volumes of water to remove any residual chemical material, clean wounds with a
mild disinfectant soap and water. Loose, nonviable tissue should be removed by
gentle cleansing with surgical soap or formal skin debride-ment. Intravenous
analgesia may be required.
·
Removal and debridement of closed
blisters is controversial. Current consensus is that intact blis-ters prevent
pain and dehydration, promote healing, and allow motion; therefore, blisters
should be left intact until they rupture spontaneously or healing is well
underway, unless they are extremely large or inhibit motion.
·
Prophylactic topical antibiotic
therapy with silver sulfadiazine is recommended for all burns except
superficial partial thickness (first-degree) burns. For first-degree burns
bacitracin may be used, but effectiveness is not documented.
·
Depending on the site and area, the
burn may be treated open (face, ears, or perineum) or covered with sterile
nonstick porous gauze. Alternatively, a petrolatum fine-mesh gauze dressing may
be used alone on partial-thickness burns. Daily dressing changes are indicated
if a burn cream is used; changes every 3 to 4 days are adequate with a dry
dressing.
·
Analgesics such as paracetamol with
codeine may be used for pain relief if needed.
·
Phosphorus particles in dermal burns
can be visualised by employing the use of Wood’s lamp.
·
Phosphorus will fluoresce under
ultraviolet light.
·
With the exposed areas immersed in
water, loose or embedded phosphorus particles that are visualised under UV
light can be mechanically but delicately removed safely under water. This
technique may be a safer alternative than either the use of copper sulfate or
silver nitrate, and may be the method of choice.
Chronic Poisoning:
·
Removal of patient from source of
exposure.
·
Dental treament and follow-up.
Autopsy Features
· Garlicky odour in the vicinity of
the mouth and in the gastric contents.
· Jaundice.
· Bleeding points in the skin
(subcutaneous haemorrhages).
· Luminous gastric contents.* The
contents will fluoresce under UV light.
· Congestion and inflammation of
affected skin and mucosa.
· Enlarged fatty liver. Later there is
evidence of aute yellow atrophy. Histopathological examination may reveal features
of acute fulminant hepatitis: collapsed reticulin framework, with fibrosis
between the hepatocytes showing bubbly, vacuolated cytoplasm.
· There may also be fatty degeneration
of heart and kidneys.
· Viscera for chemical analysis must
be preserved in satu-rated saline and not rectified spirit, otherwise
luminosity especially of the stomach contents will be lost.
Forensic Issues
·
Accidental
poisoning: This used to be common in the pastbecause of unrestricted
use of phosphorus in matches and fireworks. Today, most cases of accidental
poisoning result from inadvertent ingestion of cockroach or rat poison by
children, or because of contamination of food by these substances.
·
Suicidal
poisoning: This was also previously quitecommon, especially in Western
countries. A popular method appears to have been to soak several “lucifer”
match heads in water or brandy, mix with sugar, and consume the resultant
potion. Today, rat pastes containing phosphorus are occasionally implicated in
suicidal inges-tions.![]()
·
Homicidal
poisoning: Formerly, phosphorus was quitefrequently employed for
committing murder. Several accounts are mentioned in the literature where
poisoning was accomplished by mixing phosphorus in soup, jam, or rum, and
administered to unsuspecting victims.
Related Topics