Chapter: Modern Medical Toxicology: Neurotoxic Poisons: Anticonvulsants and Antiparkinsonian Drugs
Phenytoin - Anticonvulsant (Anti-Epileptic) Drug
Phenytoin
Synonym
Diphenylhydantoin.
Uses
■■Phenytoin is effective against all types of partial and
tonic-clonic seizures, but not absence seizures. It is NOT indicated as first
line therapy of febrile, hypoglycaemic, or other metabolic seizures.
■■Phenytoin is also used in the treatment of ventricular
arrhythmias (especially digitalis-induced).
■■It may be effective in treating paroxysmal choreoathetosis,
especially the kinesigenic type.
■■It is used alone or with other anticonvulsants to control
paroxysmal pain in some patients with trigeminal neuralgia (tic douloureux).
■■ Fosphenytoin,
mephenytoin, and ethotoin are other hydantoin anticonvulsants which are
chemically related to phenytoin. The latter two are rarely used on account of
serious adverse effects. Intravenous fosphenytoin loading has been used to
treat quinidine-like conduction defects, bradyarrhythmias, or heart block, in
tricyclic antidepressant overdose.
Toxicokinetics
·
Phenytoin is reasonably well
absorbed on oral administration. It is given intravenously in status
epilepticus.* Intramuscular injection is not advisable since the drug gets
precipitated at the site, and absorption is slow as well as erratic.
·
Phenytoin is rapidly distributed
into all tissues. The volume of distribution is 0.5 to 0.8 L/kg. It is
extensively bound to albumin (more than 90%).
·
The major metabolite resulting from
breakdown in the hepatic endoplasmic reticulum is a parahydroxyphenyl
derivative which is inactive and is excreted initially in the bile, and later
in the urine, after conjugation with glucuronide. Metabolism occurs primarily
in the liver by para-hydroxy- lation to 5-(p-hydroxyphenyl)-5-phenyl-hydantoin
(HPPH).
·
In acute overdose, peak levels are
frequently delayed for 24 to 48 hours, and occasionally as long as 7 days.
·
Protein-binding of phenytoin is impaired
in the following situations : neonatal and elderly patients, late stages of
pregnancy, hyperbilirubinaemia, liver disease, uraemia, burns, surgery,
malnutrition (and other conditions causing hypoalbuminaemia), as well as
combination therapy with salicylates, sulfonamides, valproic acid, and
tolbutamide. In these settings, the total plasma concentration of pheny- toin
may result in underestimation of the free fraction and inadvertent phenytoin
toxicity. Therefore the exact deter- mination of the free phenytoin fraction is
essential, which can be accomplished by serum ultrafiltration followed by gas
chromatography or EMIT technology.
·
At therapeutic levels, elimination
follows first-order kinetics.
Elimination half-life has been reported to be 7 to 60 hours. In overdose
settings, saturation of the hepatic hydroxylation system occurs, and zero order
kinetics predominate. Elimination follows a Michaelis-Menten model, with a
prolonged t(1/2). As phenytoin is continually excreted, elimination changes from
zero-order to first-order kinetics and drug levels decrease more rapidly.
Toxicity may persist for 7 to 10 days.
Mode of Action
·
Phenytoin acts on the motor cortex
preventing the spread of seizure activity. It stabilises the threshold for
hyperexcit- ability and reduces post-tetanic potentiation at synapses.
·
Further, phenytoin reduces brainstem
activity which is responsible for the tonic phase of tonic-clonic convulsion.
The toxic cardiovascular effects of phenytoin injection are related to the
diluent, propylene glycol. Propylene glycol is not a diluent in the current
fosphenytoin injectable forms.
Adverse Effects
·
prolonged period of time) can result in dyskinesia,
peripheral neuropathy, hirsutism, hypokalaemia, macrocytic anaemia, hepatitis,
thyroiditis, skin rashes, vertigo, mild sedation and ataxia, dysarthria,
hyperreflexia, nystagmus, and diplopia.
·
Chronic high blood levels may cause a “phenytoin
encephalopathy” with increase in seizure frequency and the development of more
tonic or opisthotonic components. Transient focal neurologic signs such as
hemiparesis may be seen, especially in brain-damaged patients.
·
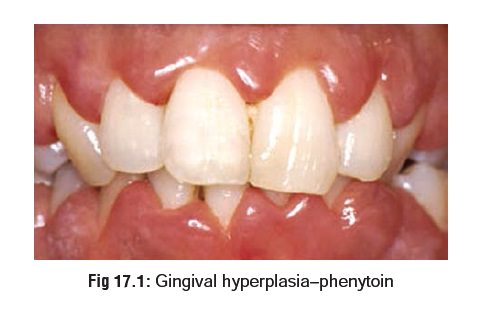
Gingival hypertrophy occurs in about 20% of patients (mostly
children or adolescents) (Fig 17.1),
and is due to altered collagen metabolism.
·
Subcapsular cataract formation has been reported in chronic
use with phenytoin.
·
Toxic hepatitis may occur leading to liver necrosis and
chronic inflammation along with cholangitis, with chronic phenytoin use.
·
Renal dysfunction, possibly a hypersensitivity reaction to
phenytoin, has also been reported with therapeutic use.
· Hypersensitivity reactions are known to occur with pheny- toin and usually manifest as fever, rash, lymphadenopathy, and hepatosplenomegaly, 3 weeks to 3 months after initia tion of therapy. Such a reaction (phenytoin hypersensitivity syndrome) can be potentially fatal.
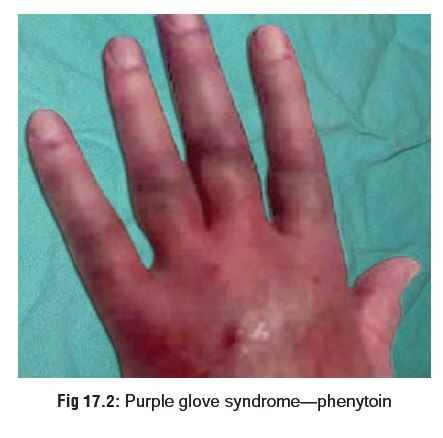
· Purple glove syndrome has been reported followingintravenous phenytoin. It is a progressive develop-ment of limb oedema, discolouration, and pain after phenytoin administration (Fig 17.2), with some patients developing skin ulceration. Elderly patients and patients receiving large, multiple IV doses appear to be at greatest risk.
![]()
· Injectable phenytoin is very alkaline
in nature with a pH of 12, and is therefore quite irritating to soft tissues if
it extravasates during IV infusion. Cyanosis and oedema have occurred in some
patients. Necrosis necessitating amputa-tion has also occurred from
extravasation of undiluted intravenous phenytoin.
· Arrhythmias and hypotension are
associated with rapid intravenous infusions of phenytoin, and appear to be due
to the diluent, propylene glycol.
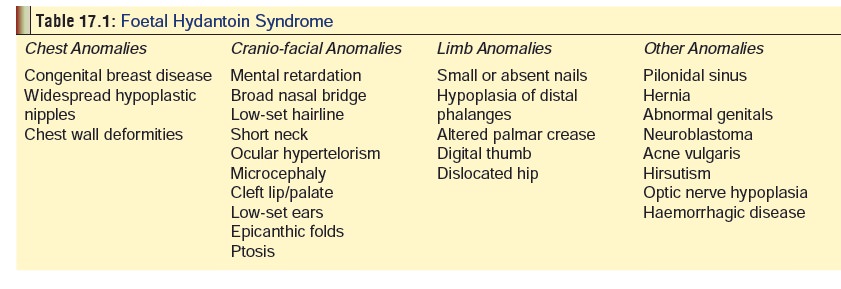
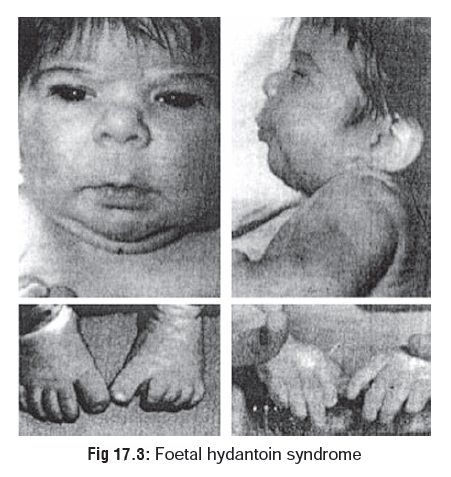
· Phenytoin is a known teratogen and
can cause a number of defects in the newborn, together referred to as foetalhydantoin syndrome, (Table 17.1) (Fig 17.3). Severalstudies have reported a possible risk of birth
defects with phenytoin, when used during the first trimester of pregnancy.
Increased risk of neural-tube defects, cardio-vascular defects, oral clefts,
and urinary tract defects have been reported. Phenytoin has also been linked
with transplacental tumorigenesis of the kidney, ureter, and bladder, and
neuroblastoma.
Drug Interactions
·
When valproic acid is administered
with phenytoin, the concentration of free phenytoin may decrease, remain
constant, or increase from pre-valproic acid levels.
·
Disulfiram has been reported to
rapidly increase serum phenytoin concentration within four hours of the admin-
istration of the first dose of disulfiram.
·
Phenytoin serum concentration has
been reported to increase to a toxic level following the addition of fluvox-
amine to the treatment regimen.
·
Ethanol can increase phenytoin serum
levels.
· Phenobarbitone may increase or decrease phenytoin levels.
·
Phenytoin serum levels have been
reported to decrease significantly when ciprofloxacin was added to the therapy
regimen.
·
Phenytoin increases the metabolism
of oestrogen and the production of sex hormone-binding globulin, via
hydroxylation, thus decreasing the effectiveness of the contraceptives.
·
Fluconazole, tolbutamide, cimetidine,
ranitidine, INH, and amiodarone have been implicated in inducing phenytoin
toxicity.
Clinical (Toxic) Features
· Overdose with phenytoin causes at
first lateral gaze nystagmus, ataxia, and drowsiness, followed by vertical or
horizontal nystagmus, oscillopsia (a very fine vertical or horizontal periodic
dancing of the eyes), slurred speech, lurching gait, coarse tremor of the
extremities, mental confusion, and disorientation. Hypothermia can occur. True
coma is uncommon.
·
Hypotension appears to be common following intravenous
phenytoin, and is concentration- and dose-related. Atrial and ventricular
conduction depression and ventricular fibril-lation have been reported
following high-dose infusionsof phenytoin. These effects are more typically
reported in elderly or gravely ill patients.
· Severe poisonings may rarely result
in respiratory depres-sion. Sometimes, paradoxical intoxication occurs
charac-terised by increased seizure activity.
· Death is rare, and if it does occur
it is invariably due to cardiac arrest or ventricular fibrillation.
Usual Fatal Dose
· Toxic
effects are rare at plasma levels less than 20 mcg/ ml (80 mcmol/L), but are common
in patients with plasma levels greater than 30 mcg/ml (120 mcmol/L).
· Estimates of the minimal lethal dose
are unreliable. Deaths are very rare even with massive acute oral overdosage
and have been reported mostly with the relatively serious hypersensitivity
reactions seen with chronic use. The lowest published lethal dose in a human
child is 100 mg/kg.
Treatment
·
Decontamination:
Stomach wash and activated charcoalare beneficial.
·
Stabilisation:
o
Cardiac monitoring is advisable following parenteral
overdose or rapid infusions.
o
Treat hypotension by Trendelenberg position, fluid
infu-sion, and pressor amines (dopamine or noradrenaline).
o
Treat heart block with atropine or pacemaker.
o
For seizures (paradoxical intoxication), use diazepam. If
seizures persist or recur, administer phenobarbitone.
·
Supportive
measures:
o
Hypersensitivity reactions may respond to corticoster-oids.
o
Forced diuresis, peritoneal dialysis, and haemodialysis are
generally ineffective. However, there are some reports on the successful use of
charcoal haemoperfu-sion in the treatment of phenytoin overdose.
Related Topics