Chapter: Modern Pharmacology with Clinical Applications: General Anesthesia: Intravenous and Inhalational Agents
Pharmacokinetic Characteristics Influencing the Clinical
PHARMACOKINETIC
CHARACTERISTICS INFLUENCING THE CLINICAL APPLICATION OF INTRAVENOUS AND
INHALATIONAL ANESTHETICS
Intravenous anesthetics are generally employed to in-duce anesthesia, to provide supplemental anesthesia, or to permit anesthesia for short operative procedures. The inhalational anesthetics are most often used for longer-term maintenance of the anesthetic state. Although in-travenous (IV) agents produce anesthesia rapidly, most are metabolized slowly, so recovery may be prolonged when an IV anesthetic is used as the primary drug dur-ing a long surgical procedure. In contrast, while the anesthetic partial pressure of an inhalational agent is achieved slowly, the patient recovers at a clinically ac-ceptable rate.
Distribution of Intravenous Drugs
Intravenously administered
anesthetic drugs are espe-cially well suited to accomplish the first
requirement of anesthetic management, rapid induction of uncon-sciousness.
These compounds generally induce anesthe-sia within one or two circulation
times after their ad-ministration because they rapidly achieve initial high
concentration in the central nervous system (CNS). These drugs enter the brain
because they are quite lipid soluble and consequently diffuse rapidly through
all bi-ological membranes, including the blood-brain barrier. In addition,
since the brain tissue receives a large pro-portion of the cardiac output, a
large proportion of an intravenously administered anesthetic will be
distrib-uted to the CNS. Tissues with lower blood flow per unit mass will
receive and therefore remove proportionally less anesthetic during the initial
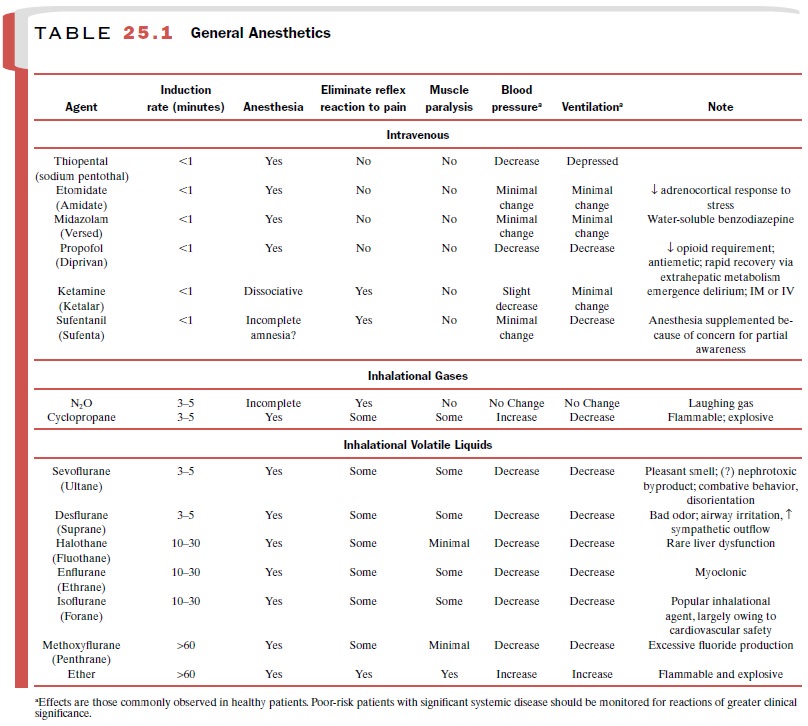
phase of drug distribu-tion. This concept is illustrated for thiopental in Fig.
25.1. All IV anesthetic drugs in use show this early pat-tern of distribution.
The use of IV anesthetics permits the patient to pass rapidly through the
initial stages of anesthesia, and sleep is induced quickly.
The initial unequal
tissue–drug distribution cannot persist, however, because physicochemical
forces tend to require an eventual establishment of concentration equilibria
with other less well perfused organs. Therefore, as the drug continues to be
removed from the blood by the less richly perfused tissues or elimi-nated by
metabolism and excretion or both, plasma lev-els will fall, and the
concentration of anesthetic in the brain will decline precipitously.
Tissues with an intermediate
blood flow per unit of mass, such as skeletal muscle and skin, are among the
first to participate in drug redistribution. In fact, it is the patient’s
skeletal muscle tissue groups that will contain the largest proportion of the
initial dose of anesthetic when the patient awakens (Fig. 25.2). Most of the IV
drugs used to induce anesthesia are slowly metabolized and excreted and depend
on redistribution to terminate their pharmacological effects. The rate of
initial redistri-bution following the administration of a single IV bolus of
drug is defined by the half-life (t1/2 ), and is generally about 8
minutes for most anesthetics. It can be said, therefore, that redistribution of
IV anesthetics to skele-tal muscle accounts for the return to consciousness
after a single sleep dose of these agents. Patients generally awaken 15 to 30
minutes after a single IV injection of most of the commonly used IV
anesthetics.
Poorly perfused tissues
(adipose tissue, connective tissue, and bone) require hours to come into
equilib-rium with plasma drug concentrations (Fig. 25.1). Since the
accumulation of anesthetic in body fat is relatively small soon after its IV
administration, it is common clin-ical practice to calculate drug dosage on the
basis of lean body mass rather than on total body weight. Thus, an obese
patient may receive the same dose of IV anes-thetic as a patient of normal body
weight.
Since the distribution of blood flow is the dominant factor controlling both tissue drug levels and the accu-mulation of IV anesthetics, changes in cardiac output can be expected to influence the pharmacological ef-fects of the IV anesthetics. Because blood flow to the brain is preserved, a greater proportion of the total dose of anesthetic will be delivered to the brain during times of diminished cardiac output, such as in congestive heart failure or hemorrhage. At such times, smaller doses of anesthetic must be administered to avoid ex-cessive CNS depression.
The use of significantly lower
doses of IV anesthetic drug also should be a considera-tion in elderly
patients, since they have low cardiac out-put, low lean body mass, and
frequently a reduced ca-pacity for drug clearance.
The effect of increased
cardiac output on the ad-ministered dose of anesthetic is opposite that
discussed for reduced cardiac output. Intensely anxious patients and those who
have such diseases as thyrotoxicosis usu-ally require larger doses of
anesthetic to induce anes-thesia.
Metabolism and Excretion of Intravenous Drugs
Clearance of IV anesthetics
from the body eventually requires metabolism and excretion. Since drugs with long elimination half-lives (t1/2 ) will have slow rates of
clearance, their use by repeated IV bolus or continuous infusion to maintain anesthesia has been restricted. Long-term
application with limited concern for the pharmacokinetics of the agents may
lead to delayed awakening, as large quantities of these drugs may accu-mulate
in reservoir tissues, such as skeletal muscle and fat. Thus, after lengthy
anesthetic administration, drug plasma levels will remain high as the compound
diffuses from these tissue reservoirs. On the other hand, if the duration of an
infusion remains short, awakening may be nearly the same with drugs exhibiting
short and long elimination half-lives. With a shorter infusion, the quan-tities
of drug accumulated in reservoir tissues are not sufficient to maintain high
plasma and brain levels. Thus, length of infusion is a context in which the
dura-tion of drug action is considered. The term context sen-sitive half-time has been coined to express the effect
of duration of infusion (the context)
on plasma levels of infused drugs.
Administration of Intravenous Anesthetics by Controlled Infusion
The recent advent of
computer-assisted IV drug admin-istration has made more practical the
maintenance of anesthesia with anesthetics with relatively short half-lives
(e.g., propofol and short-acting phenylpiperidine opioids). The technique
called total intravenous anesthe-sia (TIVA)
is done with short-acting drugs so that rapid recovery occurs even after long infusions. The loading and
maintenance doses of each agent can be pro-grammed by taking their individual
pharmacokinetic profiles into consideration. Thus, dosage is automati-cally
adjusted for factors that may alter drug distribu-tion, parameters controlling
drug clearance, and the du-ration of the procedure, to maintain a plasma level
that is just adequate for the appropriate depth of anesthesia. Many
practitioners, however, still prefer to titrate the infusion of intravenous
drugs to effect without the use of computer programming.
The shorter-acting drugs seem
to have increasing applications in outpatient surgery, which now accounts for
nearly 60% of all elective procedures, and for minor inpatient procedures
(e.g., wound repair, bronchoscopy, angiography). Patients generally receive
lower doses of drugs so that operative procedures are tolerable, avoid-ing the
substantial depression of cardiorespiratory sys-tems that may occur with the
higher doses required for hypnosis. Sedative doses of benzodiazepines and
propo-fol are among the most common; they are frequently administered in
combination with short-acting opioids. The term for such a technique is conscious sedation. Other techniques may
also be employed. For example, when an opioid is combined with a neuroleptic
drug, such as the butyrophenone droperidol (Inapsine),
the technique is called neuroleptanalgesia.
An inhalational drug, such as nitrous oxide (N2O), may be added
during intervals of the operative procedure when complete anesthesia is desired
(i.e., neuroleptanesthesia).
Related Topics