Chapter: Modern Pharmacology with Clinical Applications: General Anesthesia: Intravenous and Inhalational Agents
Mechanism of Anesthetic Action
MECHANISM OF
ANESTHETIC ACTION
Among the earliest proposals
to explain the mechanism of action of anesthetics is the concept that they
interact physically rather than chemically with lipophilic mem-brane components
to cause neuronal failure. However, this concept proposes that all anesthetics
interact in a common way (the unitary theory of anesthesia), and it is being
challenged by more recent work demonstrating that specific anesthetics exhibit
selective and distinct in-teractions with neuronal processes and that those
inter-actions are not easily explained by a common physical association with
membrane components. Proposals for the production of anesthesia are described
next.
Anesthesia from Physical Interactions with Lipophilic Membrane Components
The idea that a physical
interaction is important stems from experimental observations made in the late
nine-teenth and early twentieth centuries, when it was recog-nized that noble
gases such as xenon, which do not chemically interact with tissues, produce
unconsciousness. Also, anesthesia produced at ambient atmospheric pres-sure can
be attenuated by physically raising the pressure to 100 atm, a phenomenon known
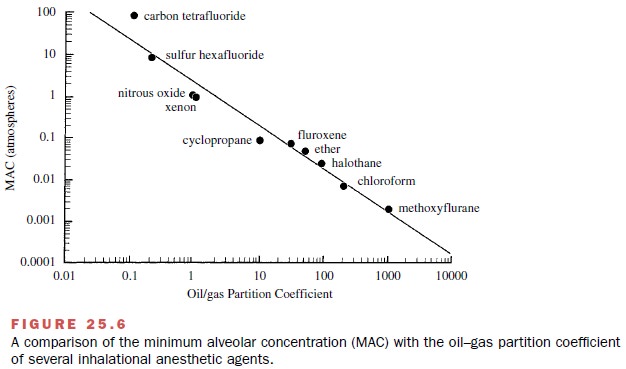
as pressure reversal. Finally, a clear correlation exists between anesthetic
po-tency and the physical parameter lipid solubility, suggest-ing that
anesthesia may be produced when anesthetics physically dissolve into the cell
membrane’s lipid bio-phase (Meyer Overton rule). Such a correlation is shown in
Fig. 25.6, where anesthetic potency is expressed as MAC and lipid solubility is
estimated as the oil–gas partition.
Membrane conformational
changes are observed on exposure to anesthetics, further supporting the
impor-tance of physical interactions that lead to perturbation of membrane
macromolecules. For example, exposure of membranes to clinically relevant
concentrations of anes-thetics causes membranes to expand beyond a critical
volume (critical volume hypothesis) associated with nor-mal cellular function. Additionally,
membrane structure becomes disorganized, so that the insertion of anesthetic
molecules into the lipid membrane causes an increase in the mobility of the
fatty acid chains in the phospholipid bilayer (membrane fluidization theory) or
prevent the interconversion of membrane lipids from a gel to a liq-uid form, a
process that is assumed necessary for normal neuronal function (lateral phase
separation hypothesis).
Anesthesia from Selective Interactions of Anesthetics with Cellular Components
While current observations do
not rule out that anes-thetics may require a hydrophobic environment near the
site of their action, they do suggest that various agents may also have
distinct interactions with tissues. For example, enantiomers of newer agents have
selec- tive and unique actions, even though they have identical physical
properties; for example, stereoisomers of isoflurane are differentially potent
but have identical oil–gas partition coefficients.
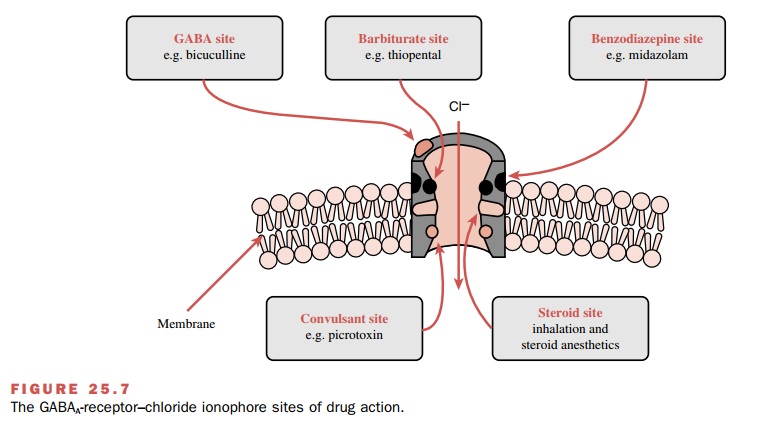
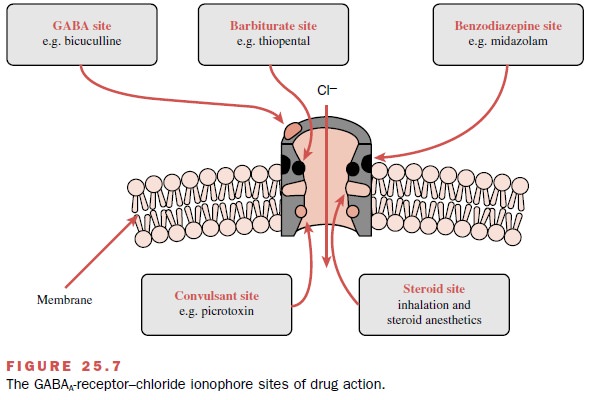
Contemporary research has
shown that at clinically relevant concentrations, various anesthetics interact
specifically with different components of the GABAA-receptor–chloride
ionophore and enhance chloride con-ductance, some directly and others by
enhancing the ac-tion of GABA. Inhalational agents directly activate the
chloride channel as well as facilitate the action of GABA, while barbiturates,
propofol, benzodiazepines, and etomidate primarily enhance the action of GABA
by interacting with specific receptor sites (Fig. 25.7). Also, anesthetics
enhance other processes known to inhibit neuronal function, such as the glycine
recep-tor–gated chloride channel. A smaller number of anes-thetics, including
ketamine, N2O, and xenon, produce neuronal inhibition by
antagonizing excitatory neu-ronal transmission mediated via the N-methyl-D-aspar-tic acid (NMDA) receptor. In addition,
some inhala-tional drugs activate K+ channels and so contribute to
hyperpolarization and reduced neuronal excitability; they also inhibit the
function of the protein complex in-volved in neurotransmitter release.
Clearly much must be
explained of the complex changes in the CNS that eventually produce
uncon-sciousness. Although physical interactions of anesthet-ics with
hydrophobic membrane components may lead to conformational changes that alter
neuronal function, specific interactions at critical receptors and ion
chan-nels are also likely to contribute to anesthesia. Thus, structurally and
pharmacologically diverse anesthetic drugs produce unconsciousness through
qualitatively different mechanisms and through actions occurring at
anatomically distinct sites in the nervous system.
Related Topics