Chapter: Modern Pharmacology with Clinical Applications: General Anesthesia: Intravenous and Inhalational Agents
Factors Affecting the Rate of Development of Anesthetic Concentration in the Lung
Factors Affecting the Rate of
Development of Anesthetic Concentration in the Lung
Gases diffuse from areas of
high partial pressure to ar-eas of low partial pressure; thus, the tension of
anes-thetic in the alveoli provides the driving force to estab-lish brain
tension. In fact, the tension of anesthetic in all body tissue will tend to
rise toward the lung tension as equilibrium is approached. Consequently,
factors that control or modify the rate of accumulation of anesthetic in the
lung (e.g., rate of gas delivery, uptake of gas from the lung into the
pulmonary circulation) will simultane-ously influence the rate at which tension
equilibria in other body compartments is established.
Graphs of the alveolar
tension plotted against time are used here to illustrate the changes in lung
partial pressure as anesthetic is inhaled. Only a fraction of total lung gases
are exchanged during one breathing cycle. Therefore, the volume of gases
already in the lung dilutes the first breath of anesthetic (breathing cycle 1
in Fig. 25.3). In subsequent breathing cycles, the alveo-lar tension will
continue to rise toward the inspired level along an exponentially declining
curve. The net change of anesthetic tension becomes smaller with each breathing
cycle, and the curve of alveolar tension will approach the inspired level more
slowly.
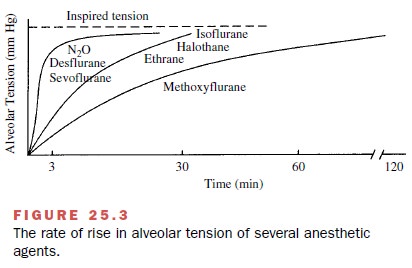
The alveolar tension–time
curve always declines in an exponential manner, but the position of the curve
can be greatly affected by the rate of delivery of anes-thetic gases and the
rate of their uptake into the pul-monary circulation. For this reason, it is
important to consider factors that modify or regulate delivery and uptake.
Effect of the Alveolar–Arterial Tension Gradient on Alveolar Tension of Anesthetic Gas
Tissues, including the brain,
that have a high blood flow per unit mass (Fig. 25.1) equilibrate with the
alveolar tension of anesthetic gases first. Tissues with lower blood flow
require a longer time and continue to accu-mulate anesthetic gas during the
maintenance phase of
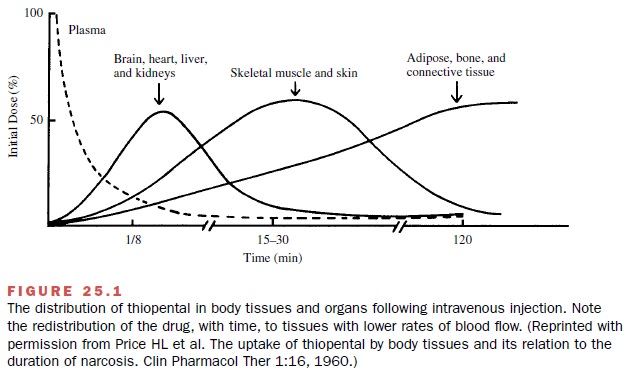
Since more blood will pass through the pulmonary capillary bed when the cardiac output is high, it follows that a greater total transfer of any anesthetic agent across the alveolus will anesthesia, that is, after patients become unconscious.
As body tissues become saturated with anesthetic
mol-ecules, blood returning to the lung will have increas-ingly high anesthetic
tension, and the alveolar–arterial tension gradient will be reduced. Since the
gradient controls the rate of diffusion across the alveolar capil-lary
membrane, uptake is also reduced and the rate of rise of the alveolar tension
of anesthetic is accelerated.
Effect of Solubility of Various Agents
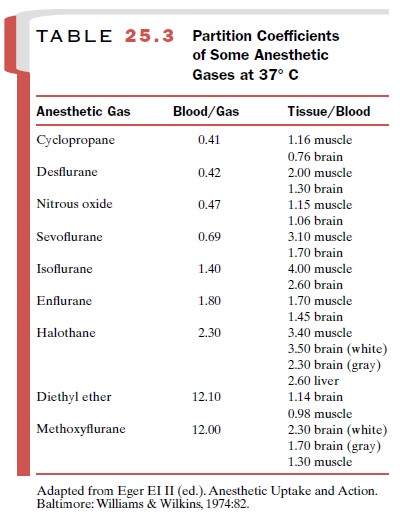
The inhalational anesthetics
have distinctly different solubility (affinity) characteristics in blood as
well as in other tissues. These solubility differences are usually expressed as
coefficients and indicate the number of volumes of a particular agent
distributed in one phase, as compared with another, when the partial pressure
is at equilibrium (Table 25.3). For example, isoflurane has a blood-to-gas
partition coefficient (often referred to as the Ostwald solubility coefficient)
of approximately 1.4. Thus, when the partial pressure has reached equi-librium,
blood will contain 1.4 times as much isoflurane as an equal volume of alveolar
air. The volume of the various anesthetics required to saturate blood is
similar to that needed to saturate other body tissues (Table 25.3); that is,
the blood–tissue partition coefficient is usually not more than 4 (that of
adipose tissue is higher).
The solubility of anesthetic
agents is a major factor for the rate of induction of anesthesia, or the time
re-quired to establish a level of unconsciousness adequate for surgery. Agents
with limited plasma solubility and a low rate of uptake (e.g., N2O,
cyclopropane, sevoflurane, and desflurane) will equilibrate rapidly with
tissues. For an agent that is highly soluble in plasma (e.g., methoxyflurane),
the rate of rise of alveolar tension to the inspired level and the equilibration
of the gas with brain will be delayed by a higher initial uptake into plasma
from the alveoli. This phenomenon is often counterintuitive to students.
However, with gases, par-tial pressure is the controlling factor for
equilibration between tissues, and even though uptake is high, partial pressure
in the tissues and lung rises slowly, as large quantities of a highly soluble
gas must be accumulated to establish the desired tension (Henry’s law).
To illustrate the effect of
solubility on the rate of induction of anesthesia, we can consider a situation
in which individual agents are delivered to patients at their equivalent MAC
values. Under these conditions, regardless of the agent being employed, a
similar level of anesthesia will be achieved. In contrast, induction rates,
illustrated as the time required for the alveolar tension to rise to the
inspired level (Fig. 25.3), can be seen to be quite different. A patient
receiving a MAC of N2O, desflurane, or sevoflurane will be
unconscious within 3 minutes. However, halothane, enflurane, and isoflurane,
which have significant blood and tissue solubilities, will require at least 30
minutes before surgical anesthesia is established. Methoxyflurane, a highly
soluble agent, requires several hours and may be clinically impractical if
administered in this way.
Effect of Pulmonary Perfusion
The rate of pulmonary
perfusion (in healthy individuals, essentially equivalent to the cardiac
output) also affects the rate of induction of anesthesia. Since more blood will
pass through the pulmonary capillary bed when the cardiac output is high, it
follows that a greater total transfer of any anesthetic agent across the
alveolus will occur in these conditions. Also, tissues normally receiv-ing a
smaller proportion of the total cardiac output re-ceive a greater amount when
cardiac output is high and will accumulate a larger proportion of the
anesthetic crossing the alveolar membrane. Ultimately, greater up-take will
slow the rate of rise of the alveolar tension– time curve, and anesthetic
induction with an individual agent may be slower when the cardiac output and
per-fusion of the lung are high. In low cardiac output states, the reverse is
true. The rate of uptake will be lower, and the alveolar tension will rise
toward the inspired tension more quickly. To minimize the effect of cardiac
output on the rate of induction of anesthesia, agents of lower solubility would
be preferred clinically.
Effect of the Rate of Ventilation and Inspired Gas Concentration
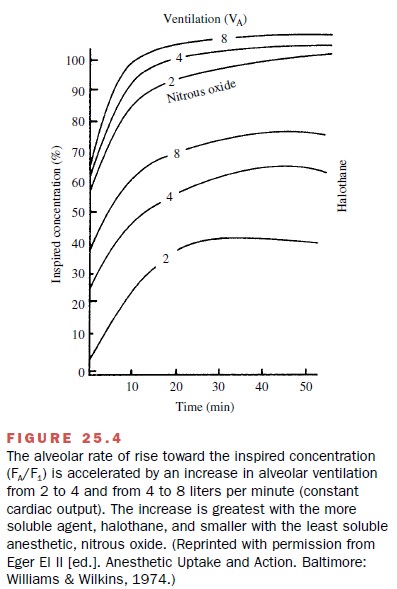
Frequently it is desirable to
overcome the slow rate of rise of alveolar tension associated with such factors
as the high blood solubility of some anesthetics and in-creased pulmonary blood
flow. Since both of these fac-tors retard tension development by increasing the
up-take of anesthetic, the most effective way to alleviate the problem is to
accelerate the input of gas to the alve-oli. A useful technique to increase the
input of anes-thetic to the lung is to elevate the minute alveolar
ven-tilation. This maneuver, which causes a greater quantity of fresh
anesthetic gas to be delivered to the patient per unit of time, is most
effective with highly soluble agents (Fig. 25.4).
Increasing the inspired
tension of an anesthetic gas above the maintenance tension (i.e., near the MAC
value) is also an effective means of quickly establishing effective alveolar
tension. This maneuver, frequently re-ferred to as overpressure, parallels the
concept of load-ing dose. As the desired depth of anesthesia or level of
alveolar tension is achieved, the delivered tension of anesthetic must be
returned to the maintenance (MAC) level to avoid overdosing the patient.
Other Factors Affecting the Alveolar Tension of Anesthetic Agents
Special factors influence the
rate of rise of the alveolar tension to the inspired level when anesthetics are
deliv-ered in high concentration. These factors particularly significant when N2O
is used, since it is often required in concentrations exceeding 25% in the
inspired air.
Concentration Effect
When anesthetics are
delivered in high concentra-tion, the alveolar tension will rise rapidly. Thus,
if 75% N2O is being delivered in the inspired air, the 75% ten-sion
in blood will be established more quickly than if 40% N2O were being
inhaled and a 40% N2O tension were desired in blood.
Second Gas Effect
The alveolar tension of other
anesthetic gases also rises more rapidly (second gas effect) when an
anes-thetic such as N2O is present in high concentration. These
gases are also subject to the increased inflow (pulling in of fresh gases) as N2O
is taken up into the blood.
Diffusion Hypoxia
Diffusion hypoxia may be
encountered at the end of an anesthetic administration with N2O. The
mechanism underlying diffusion hypoxia is essentially the reverse of the
concentration effect; that is, when anesthetic ad-ministration is stopped,
large volumes of N2O move from the blood into the alveolus, diluting
oxygen and expanding lung expiratory volume. To avoid diffusion hypoxia, the
anesthesiologist may employ 100% oxygen rather than room air after
discontinuing administration of the anesthetic gas mixture.
Related Topics