Chapter: Essential Clinical Immunology: Immunological Aspects of Gastrointestinal and Liver Disease
Pernicious Anemia - Immune Mediated Diseases of the Gastrointestinal Tract
IMMUNE-MEDIATED DISEASES OF THE GASTROINTESTINAL TRACT
Pernicious Anemia
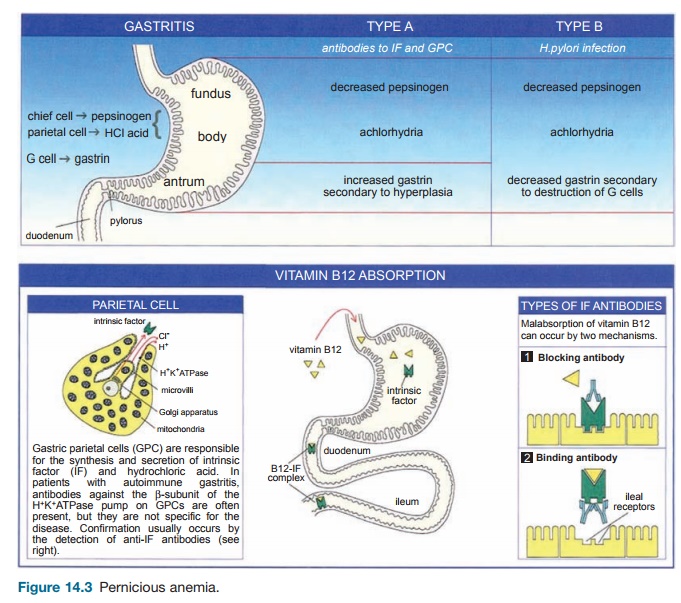
Pernicious anemia (PA) is an organ-specific autoimmune disease characterized by chronic inflammation of the stomach (gastritis) with subsequent loss of parietal cells. This loss of parietal cells results in decreased synthesis of intrinsic factor (IF), which is critical for the absorption of vitamin B12 (cobalamin) in the terminal ileum. Although there are many causes of B12 deficiency (not discussed here), it should be emphasized that the term PA is reserved for instances where a lack of B12 results specifically from a deficiency of IF in the stomach.
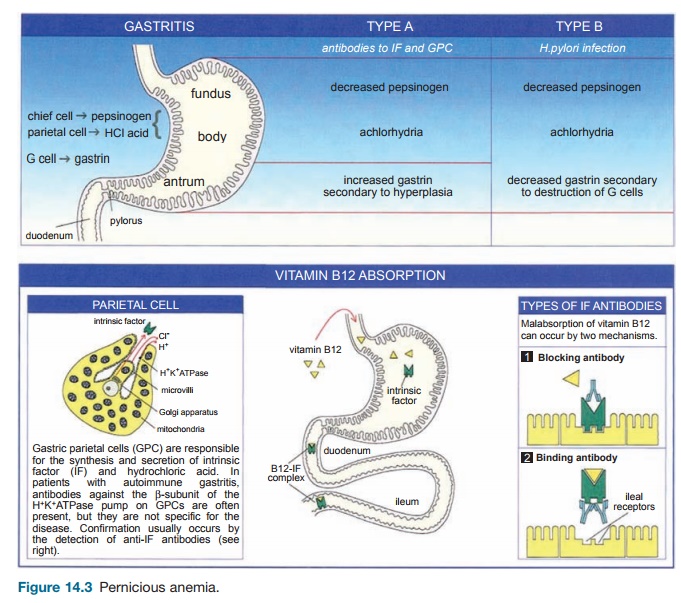
The stomach is divided into three regions: the body and fundus, which pro-duce acid and pepsinogen through the parietal cells and chief cells, respectively, and the antrum where G cells produce gas-trin (Figure 14.3). Chronic gastritis can be divided into two categories depending on the etiology. The gastritis associated with PA is commonly referred to as type A or autoimmune gastritis and is characterized by autoantibodies that are targeted against gastric parietal cells and IF in the body and fundus. The antrum is spared. This pro-cess results in decreased levels of acid and pepsinogen, but increased levels of gastrin subsequent to a loss of negative feedback inhibition of G cells from achlorhydria.
On a histologic level, there is a chronic inflammatory infiltrate in the LP com-posed of mononuclear cells, plasma cells, T cells, and B cells. The plasma cells pro-duce the autoantibodies directed against gastric parietal cells and IF. Persistent chronic inflammation with involvement of the mucosa results in the degeneration of parietal and chief cells. In advanced lesions, there is replacement of these cells by mucus-secreting cells referred to as intestinal metaplasia. In contrast to type A gastritis, type B (or nonautoimmune) gastritis is the result of Helicobacter pylori infection that involves the entire stomach, including the antrum (gastrin levels are decreased).
PA is indolent in its course and may take as long as twenty to thirty years for the disease to progress from its histologic findings of gastritis to that of gastric atro-phy and clinical anemia. As many as 2 percent of individuals greater than sixty years old have PA but go undiagnosed. At one time, PA was thought to be preva-lent predominantly in northern European populations, but the disease has also been found to affect Latin American and black populations as well. Although no asso-ciations with specific haplotypes have been found, the disease tends to cluster in families, suggesting a genetic compo-nent. In addition, there is a predisposition of individuals who have PA to also have other autoimmune endocrinopathies such as autoimmune (Hashimoto’s) thyroiditis, Addison’s disease, and insulin-dependent diabetes mellitus.
Great progress in understanding PA was made with the discovery that the target of the gastric parietal cell (GPC) autoantibod-ies was H+/K+ ATPase, an enzyme present in the secretory canaliculi. In vitro, anti-GPC antibodies are capable of activating complement and lysing cells, but it is unlikely that these antibodies are the cause of PA, given their limited accessibility to the intracellularly situated H+/K+ ATPase. Instead, research suggests that the primary lesion is initiated by CD4+ T cells that rec-ognize the β subunit of H+/K+ATPase. The best-characterized murine model in the study of autoimmune gastritis is that of neonatal thymectomized mice that develop gastritis. However, when transgenic mice induced to express the β subunit of H+/K+ ATPase in the thymus are thymectomized, they do not develop gastritis. The implica-tion here is that exposure to the β subunit of H+/K+ ATPase in the thymus early on in life may be critical in the induction of some regulatory cell population that is necessary to prevent gastritis or that autoreactive cells are deleted.
Diagnosis of vitamin B12 deficiency has traditionally been based on low serum vitamin B12 levels along with clinical evi-dence of disease (most commonly mega-loblastic anemia). However, some studies have shown that increased levels of meth-ylmalonic acid and homocysteine, key metabolites in the enzymatic reactions that require vitamin B12, are more sensitive for the diagnosis of vitamin B12 deficiency. The presence of anti-GPC autoantibodies is very sensitive but lacks specificity as they can occur in other autoimmune states. In contrast, antibodies to IF have 50 percent sensitivity, but are fairly specific.
Normally, upon intake, vitamin B12 forms a complex with IF in the duodenum and the B12–IF complex is subsequently taken up by receptors in the distal ileum for absorption. Two types of anti-IF auto-antibodies – blocking and binding – have been found to interfere with this process in PA. Blocking antibodies prevent the attach-ment of B12 to IF while binding antibodies are thought to prevent the adhesion of the complex to ileal receptors.
Intramuscular injection of vitamin B12 has been the mainstay of treatment for PA. However, clinical trials now suggest that in individuals who do not show signs of neurologic involvement (even those indi-viduals with IF deficiency), oral therapy may be considered as an alternative. This is supported by studies that demonstrate B12 absorption may also occur by a pathway independent of IF. Treatment is impor-tant to prevent anemia and neurologic sequelae.
Related Topics