Chemistry in Daily Life | Term 3 Unit 4 | 7th Science - Oral Rehydration Solution (ORS) | 7th Science : Term 3 Unit 4 : Chemistry in Daily Life
Chapter: 7th Science : Term 3 Unit 4 : Chemistry in Daily Life
Oral Rehydration Solution (ORS)
Oral Rehydration Solution (ORS)
ORS
(Oral Rehydration Solution) is a special combination of dry salts that is mixed
with safe water. It can help to replace the fluids lost due to diarrhea. In a
state of diarrheal disease there is imbalance and much more water is secreted
than reabsorbed causing a net loss to the body which can be as high as several
liters a day. In addition to water loss, sodium and potassium are also lost.
Certain
concentration of sodium (Na) is needed for proper functioning of the body.For example, only with adequate
sodium concentration in the intestinal wall, water can be absorbed by it
through a process known as osmosis. If there is inadequate salt in the
intestinal wall the body will not be able to absorb water.
The saline bottle directly transfers
water and sodium into the blood stream. However, for the saline water is
administered through mouth, intestinal wall, is a not able to absorb neither
water nor sodium. Dr. Dilip Mahalanabis found that if glucose (sugar) is added
to the salt solution, then all the three- water, sodium and glucose are
absorbed by the body.
During diarrhea the intestine is
still able to absorb glucose molecules. Thus, the ORS solution uses the glucose
molecules to enable the sodium to be is carried through by a co-transport
coupling mechanism. ORS is an effective treatment for 90 – 95% of patients
suffering from diarrhea, regardless of the cause. As the water is replaced
balance is attained saving the patience in most cases.
Let us see homely made of ORS, be
very careful to mix 6 level teaspoons of sugar and 1/2 level teaspoon of salt
dissolved in 1 litre of clean water. Too much sugar can make diarrhea worse.
Too much salt can be extremely harmful to the child. Making the mixture a
little too diluted (with more than 1 litre of clean water) is not harmful.
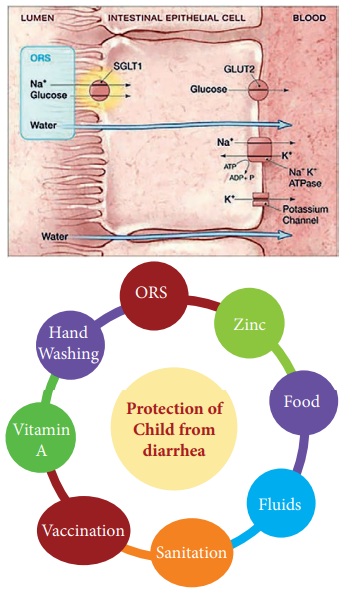
Through the process of osmosis, the
salts and sugars pull water into your bloodstream and speed up rehydration.
Related Topics