Chemistry in Daily Life | Term 3 Unit 4 | 7th Science - Antacid | 7th Science : Term 3 Unit 4 : Chemistry in Daily Life
Chapter: 7th Science : Term 3 Unit 4 : Chemistry in Daily Life
Antacid
Antacid
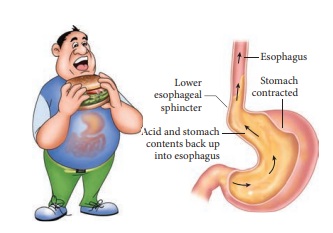
Acidity is a set of symptoms caused
by excess production of acid by the gastric glands of the stomach. Your stomach
naturally produces gastric or hydrochloric acid (HCl) to help digest and break
down food. Acidity issues arise when there is excess production of this acid
due to triggers such as acidic foods, spicy food, alcohol, dehydration and
stress. When acidity occurs, the excess acid may move up from your stomach to
your esophagus.
The lining of your stomach with a pH
of 1 to 3 is designed as such to withstand a high acidic environment.
When we have acidity or heartburn,
we are administered a class of medicines known as antacids. They are actually
weak bases. As learned in chemistry, when a base is mixed with an acid a
neutralization reaction occurs. When antacids are consumed, it creates a
chemical reaction in the stomach lowering the acidity and makes the digestive
acids less corrosive and damaging.
Most of the common antacids are
Sodium Bicarbonate (NaHCO3), Calcium Carbonate (CaCO3),
Magnesium Hydroxide (Mg (OH)2), Magnesium Carbonate (MgCO3)
and Aluminium Hydroxide Al(OH)3.
The chemical reaction created when
Magnesium Hydroxide neutralizes HCI in the stomach and intestine
Related Topics