Chapter: Essentials of Psychiatry: Obsessive-Compulsive Disorder
Obsessive-Compulsive Disorder: Treatment, Pharmacological Treatments, Assessing Treatment Resistance
Treatment
General Considerations
Both pharmacologic and behavioral therapies have proved effec-tive for
OCD. The majority of controlled treatment trials have been performed with
adults age 18 to 65 years. However, these therapies have been shown effective
for patients of all ages. In general, children and the elderly tolerate most of
these medi-cations well. For children, lower doses are indicated because of
lower body mass. For instance, the recommended dose for clomipramine in
children is up to 150 mg/day (3 mg/kg/day) versus 250 mg/day in adults. Use of
lower doses should also be considered in the elderly because their decreased
ability to metabolize medications can increase the risk of side effects and
toxicity. Behavioral therapy has also been used successfully in all age groups,
although when treating children with this mo-dality it is usually advisable to
use a parent as a cotherapist. A flowchart that outlines treatment options for
OCD is shown in Figure 51.5
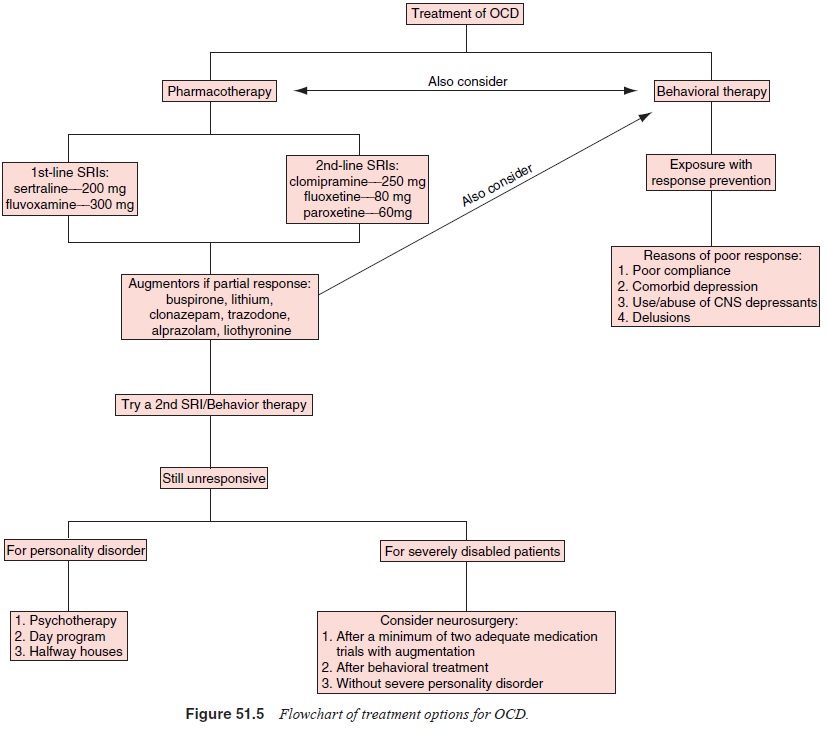
In general, the goals of treatment are to reduce the fre-quency and
intensity of symptoms as much as possible and to minimize the amount of
interference the symptoms cause. It should be noted that few patients
experience a cure or complete remission of symptoms. Instead, OCD should be
viewed as a chronic illness with a waxing and waning course. Symptoms are often
worse during times of psychosocial stress. Even when on medication, individuals
with OCD are often upset when they experience even a mild symptom exacerbation,
anticipating that their symptoms will revert to their worst, which is rarely
the case. Anticipating with the patient that stress may make the symptoms worse
can often be helpful in long-term treatment. Expert consensus guidelines, based
on completion of a survey by 79 experts in the field, provide a reasonable
approach to clini-cal practice in treating patients with OCD. However like any
consensus report, based on clinical practice, not all of the rec-ommendations
are supported by empirical data (March et
al., 1997). Further work in neurosurgical techniques, particularly less
invasive approaches like gamma knife and possibly trans-magnetic stimulation,
may offer other options in the future for treating OCD.
Pharmacological Treatments
The most extensively studied agents for OCD are medications that affect
the serotonin system. Many studies implicate the serotonin system in OCD’s
pathophysiology, although comparative studies also seem to implicate other
neurotransmitter systems, including the dopaminergic system, in treatment
response. The principal pharmacologic agents used to treat OCD are the SRIs,
which in-clude clomipramine, fluoxetine, fluvoxamine, sertraline, paroxet-ine
and citalopram.
Outcome measures in OCD treatment trials generally in-clude the Yale-Brown Obsessive–Compulsive Scale (Y-BOCS; Goodman et al., 1989), a reliable and valid 10-item, 40-point semi-structured instrument that assesses the severity of obses-sions and rituals during the preceding week. Studies conducted since 1989 have generally used the Y-BOCS as one of the major outcome measures. Most studies have used Y-BOCS scores of 16 to 20 as a study entry criterion, although it has been argued that higher scores (e.g., 20–21) might reduce the increasing pla-cebo response rates being obtained in OCD studies. Treatment response is generally considered to constitute at least a 25 to 35% reduction in OCD symptoms as measured by the Y-BOCS. Another frequently used global outcome measure is the National Institute of Mental Health Global Obsessive–Compulsive Scale (NIMH-OC) (Pato et al., 1994).
Clomipramine
The tricyclic antidepressant clomipramine is among the most extensively
studied pharmacological agents in OCD. This drug is unique among the
antiobsessional agents in which in addition to its potency as an SRI, it has
significant affinity for noradren-ergic, dopaminergic, muscarinic and
histaminic receptors. The most common side effects are those typical of the tricyclic
anti-depressants, including dry mouth, dizziness, tremor, fatigue, somnolence,
constipation, nausea, increased sweating, head-ache, mental cloudiness and
sexual dysfunction. Previous data have indicated that at doses of 300 mg/day or
more, the risk of seizures is 2.1% but at doses of 250 mg/day or less, the risk
of seizures is low (0.48%) and comparable to that of other tri-cyclic
antidepressants. It is therefore recommended that doses of 250 mg/day or less
be used. Elderly patients may be more prone to tricyclic side effects, such as
orthostatic hypotension, constipation (which may lead to fecal impaction),
forgetfulness and mental cloudiness, which might be confused with dementia.
Most of these side effects can be treated by simply lowering the dose, although
the cardiac conduction effects of tricyclic anti- depressants may preclude the
use of clomipramine in patients with preexisting cardiac conduction problems,
especially atrio-ventricular block.
Recent studies of IV clomipramine have been par-ticularly promising
because it seems to have a quicker on-set of action and fewer side effects than
the oral form, and it may be effective even in patients who do not respond to
oral clomipramine. Oral clomipramine, like other SRIs, usu-ally takes a minimum
of 4 to 6 weeks to produce a clinically significant clinical response, but in
at least one study using IV pulse doses patients showed a response within 4.5
days. The reasons for this unique response are not fully understood, but it is
postulated that the IV preparation avoids first-pass hepatoenteric metabolism,
leading to increased bioavailabil-ity of the parent compound clomipramine. This
in turn may play a role in rapidly desensitizing serotonergic receptors or
initiating changes in postsynaptic serotonergic neurons. This preparation is
still not FDA-approved for clinical use in the USA. Cardiac monitoring is
recommended during the use of IV clomipramine.
Fluoxetine
Despite their different chemical structures, all of the SSRIs appear to
have similar efficacy in treating OCD. Fluoxetine has not been shown to be more
effective than clomipramine. The fixed-dose trials of fluoxetine are
particularly noteworthy because there are few published fixed-dose trials with
any of the antiobsessional agents in OCD. Although these studies indicated that
doses of 20, 40 and 60 mg/day were all effective when compared with pla-cebo,
there was a trend toward 60 mg/day being more effective. Some patients who did
not respond at lower doses responded at higher doses, and others who responded
at lower doses showed increased improvement at a higher dose. In addition,
patients maintained their improvement or experienced increased im-provement during
the 5- to 6-month follow-up period.
Fluoxetine has fewer side effects than clomipramine, re-flecting its
more selective mechanism of action. The most com-mon side effects are headache,
nausea, insomnia, anorexia, dry mouth, somnolence, nervousness, tremor and
diarrhea. Side ef-fects occur more frequently at higher doses. Fluoxetine’s
long half-life, which is unique among the SRIs, is 2 to 4 days for the parent
compound and 4 to 16 days for its active metabolite. This long half-life can be
beneficial for patients who do not comply with treatment, because relatively
high steady-state levels are maintained even when several doses are missed.
However, the long half-life can present problems when switching or
discon-tinuing fluoxetine, because 5 weeks or more may be required for the
medication to be completely cleared from the body. Hence the added delay, 5
weeks rather than 2 weeks for the other SRIs, is required when switching from
fluoxetine to an MAOI.
Fluvoxamine
Fluvoxamine is a unicyclic agent which differs from the other SSRIs in
that it does not have an active metabolite. A number of systematic blinded
clinical trials, most of which were placebo-controlled, have demonstrated
fluvoxamine’s effectiveness in treating OCD. As has been demonstrated for the
other SSRIs, relatively long treatment trials are indicated before concluding
that an SSRI is ineffective in OCD.
The largest and most recent fluvoxamine study was a 10-week multicenter
double-blind placebo-controlled study of 320 patients. The mean dose of
fluvoxamine was 249 mg/day. As in the other studies, the fluvoxamine-treated
patients had a sig-nificant reduction in OCD symptoms; however, unlike most of
the earlier medication trials there was a relatively high placebo response rate
of 11%. One of the important conclusions from this study was that prior failure
to respond to another SSRI was associated with a lower likelihood of responding
to fluvoxamine. All of the fluvoxamine studies show a similar side-effect
pro-file, which included insomnia, nervousness, fatigue, somnolence, nausea,
headache and sexual dysfunction. Insomnia and nerv-ousness tended to occur early
in treatment, whereas fatigue and somnolence occurred later. Overall, the
medication is well toler-ated, with only 10 to 15% of patients dropping out of
treatment because of side effects.
Sertraline
As is true of other studies, higher doses of sertraline 200 mg/day being
is more effective than 50 mg/day or 100 mg/day. Typical side effects included
nausea, headache, diarrhea, insomnia and dry mouth. A recent study compared
sertraline with the non-SRI antidepressant desipramine for patients with OCD and
co-morbid depression. Although these medications were similarlyefficacious for
depression, sertraline was more effective for OCD symptoms, supporting the use
of an SRI-like sertraline rather than a norepinephrine reuptake inhibitor like
desipramine in such patients. In addition, even though desipramine did improve
depressive symptoms, a significantly greater number of patients treated with
sertraline achieved remission from depression (Hoehn-Saric et al., 2000).
Paroxetine
Paroxetine is another SSRI that differs in structure from those
previously discussed. It is a phenylpiperidine compound that is marketed as an
antidepressant and, like sertraline, shows promise in the treatment of OCD.
Results of a fixed-dose multicenter trial of 348 patients indicated that
paroxetine is effective for OCD. As was suggested in the sertraline study,
higher doses (40 or 60 mg/day) may be needed because 20 mg/day was no more
effec-tive than placebo (Wheadon et al.,
1993). Paroxetine’s efficacy is comparable to that of other SRIs, and side
effects are similar to those of other SSRIs and include lethargy, dry mouth,
nausea, insomnia, somnolence, tremor, sexual dysfunction and decreased
appetite. Reports of an acute discontinuation syndrome, which can include
general malaise, asthenia, dizziness, vertigo, head-ache, myalgia, loss of
appetite, nausea, diarrhea and abdominal cramps, warrant a gradual reduction in
dose if this medication is to be discontinued. Occasional patients may
experience some of these symptoms even if their dose is delayed by only a few
hours.
Citalopram
Citalopram is the newest SSRI available for the treatment of OCD and is
unique in its selectivity for serotonin reuptake com-pared with the other SRIs.
It has few significant secondary bind-ing properties, and its minimal effect on
hepatic metabolism probably makes it safer to combine with other medications. A
multicenter fixed-dose-placebo-controlled trial with 401 patients showed that
52 to 65% of patients responded in the three dosage groups compared with a 37%
response rate in the placebo group. While there was a trend for a higher dose
to lead to a higher re-sponse rate, as with other SRIs, there was no
statistical difference between the three doses used (20, 40 and 60 mg/day).
Typical side effects included fatigue, sweating, dry mouth, ejaculation
failure, nausea and insomnia, although many patients habituated to these side
effects in 4 to 6 weeks. Thus, citalopram is a good choice for OCD treatment
because of its side-effect profile and low probability of causing drug–drug
interactions (Montgomery et al.,
2001).
Other Agents
Most studies of other medications (venlafaxine, buspirone, trazo-adone,
clonazepam and MAOIs for OCD have consisted of only case reports or small
samples. However, few of these agents have been promising enough to warrant
large blinded efficacy trials.
Which SRI to Choose?
The efficacy of each SRI – clomipramine, fluoxetine, fluvoxam-ine,
sertraline, paroxetine, and citalopram – is supported by ex-isting data. During
the last 12 years at least seven head-to-head SRI comparison studies have been
done, six of which compared clomipramine with fluoxetine, fluvoxamine,
paroxetine and ser-traline. All of the studies found that the agents studied
were equally efficacious, although they may have been underpow-ered to detect
differences among medications. However, severalmeta-analyses of OCD trials,
which compared SRIs across large placebo-controlled multicenter trials, lend
some support to the notion that clomipramine might be more effective than the
more selective agents. These meta-analyses support a trial of clomi-pramine in
all patients who do not respond to SRIs, even though clomipiramine tends to
cause more side effects.
A number of studies have assessed predictors of medica-tion response.
Predictors of poor response include failure to re-spond to a previous SRI,
early age of OCD onset, presence of schizotypal personality disorder and
presence of hoarding. How-ever, not all studies have had consistent findings,
and more inves-tigation of this issues is needed using larger and more narrowly
defined samples.
It is worth noting that the SSRIs, via their effect on the liver
cytochrome system, can inhibit the metabolism of certain other drugs.
Fluoxetine can elevate blood levels of a variety of coadministered drugs,
including tricyclic antidepressants (such as clomipramine), carbamazepine,
phenytoin and trazodone. However, the other SSRIs (with the exception of
citalopram) can theoretically cause similar elevations, although fewer reports
on such interactions are currently available. Some clinicians have taken
advantage of these interactions by carefully combining flu-voxamine with
clomipramine in order to block clomipramine’s metabolism to
desmethylclomipramine; this in turn favors se-rotonin reuptake inhibition
provided by the parent compound rather than the norepinephrine reuptake
inhibition provided by the metabolite. However, caution should be used with
this ap-proach since the elevation in clomipramine levels, and perhaps other
compounds, can be nonlinear and quickly lead to danger-ous toxicity; at the
very least, clomipramine levels should be carefully monitored.
All of the SSRIs are generally well tolerated, with a rela-tively low
percentage of patients experiencing notable side ef-fects or discontinuing them
because of side effects. In addition, these compounds are unlikely to be lethal
in overdose, except for clomipramine, which can lead to cardiac arrhythmias and
death. All these agents can cause sexual side effects, ranging from anorgasmia
to difficulty with ejaculatory function. However, such symptoms are not readily
volunteered by the patient, thus it is important to ask. Should such symptoms
be experienced, conservative measures may include dosage reduction, transient
drug holidays for a special weekend or occasion, or switching to another SRI
since patients may not have the same degree of dysfunction with a different
agent. However if the clinician feels that it is critical to continue with same
agent, various treatments have been reported in the literature. Usually taken
within a few hours of sexual activity, no one agent has been shown to work
consistently. Among those that have been tried are yohimbine, buspirone,
cyproheptadine, buproprion, dextroamphetamine, methylphenidate, amantidine,
nefazodone, to name but a few.
Assessing Treatment Resistance
Before concluding that a patient is treatment-resistant, the ad-equacy
of previous treatment trials must be assessed (see Table 51.2). In particular,
it is critical to know both the dura-tion and dose of every medication that has
been used. Typically, patients who appear treatment-resistant have received an
inad-equate duration of treatment, which should be a minimum of 10 to 12 weeks,
or an inadequate medication dose, which should be the maximum dose for any
particular agent. Some psychiatrists consider patients truly
treatment-resistant only if they have failed

several adequate pharmacologic trials, including one with clomi-pramine,
and several augmentation strategies including behav-ioral therapy. With this
kind of aggressive treatment, 80 to 90% of patients usually experience some
improvement, although few patients become symptom-free.
When inadequate treatment is not the reason for poor treatment response,
it is important to assess the accuracy of the diagnosis. Schizotypal
personality disorder, borderline personal-ity disorder, avoidant personality
disorder and OCPD seem to be associated with poorer response to
pharmacotherapy, particularly if the personality disorder is the primary
diagnosis. Behavioral therapy seems less effective in patients with comorbid
major de-pressive disorder. Comorbid depression may inhibit the ability to
learn and to habituate to anxiety. Initial pharmacotherapy some-times improves
depression, as well as OCD symptoms, and may increase the likelihood of success
with behavioral treatment.
Related Topics