Chapter: Essential Clinical Immunology: Immunological Aspects of Infection
Non Specific Resistance - Immunological Aspects of Infection
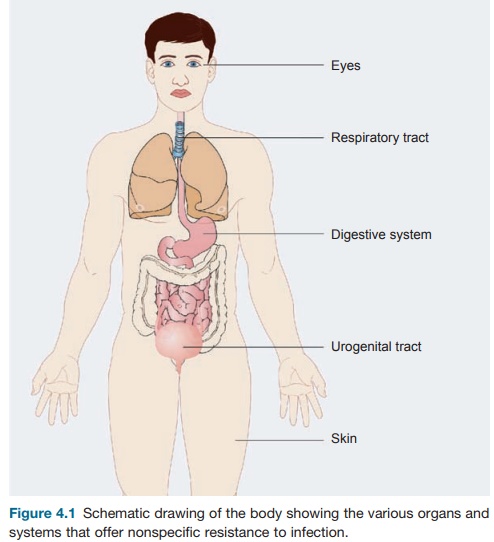
NONSPECIFIC RESISTANCE
Nonspecific or natural resistance refers to
barriers, secretions, and normal flora that make up our external defenses.
Phago-cytes and complement are also involved. Mechanical barriers are highly
effective, and the skin (our largest organ) is highly suited to this protection
(see Figure 4.1); loss of a major part of the skin (second-ary to burns, acids,
etc.) immediately exposes the host to marked susceptibility to infection. The
mucosal lining of mouth and respiratory tract is another excellent defense
mechanism. Yet, a defect in the mucosal lining of the respiratory tract, which
occurs in cystic fibrosis, results in a heightened susceptibility to many
infec-tions. These are examples of a defect in the epithelium or epithelial
lining. In gen-eral, however, it is the mobilization of the phagocytic cells
such as monocytes/mac- rophages and polymorphonuclear leuko-cytes that ingest
invading microorganisms and kill them.
The polymorpholeukocytes are a large pool of
phagocytic cells that are both cir-culatory and in the bone marrow. Invad-ing
organisms trigger an inflammatory cascade, which stimulates these cells to
adhere to vascular epithelium and actively migrate toward the infection.
Phagocytosis is promoted by opsonins
(usually IgG anti-body) and complement.
The macrophages reside in the sub-epithelial
tissues of the skin and intestine and line the alveoli of the lungs. Microbes
that penetrate an epithelial surface will encounter local tissue macrophages
called histocytes. If the organism
enters via blood or lymph, then
defense is provided by fixed macrophages called Kupffer cells, which line the sinusoids of the liver. Simi-larly
fixed macrophages called Langerhans cells are also present in the epidermis
of the skin. Once engaged with the
organ-ism, these macrophages release a number of macrophage-derived cytokines,
which nonspecifically amplify the immuno-logical and inflammatory reactions to
the invading microbe.
Most pathogenic microorganisms have evolved methods
of resisting phagocy-tosis. For example, group A streptococci have cell surface
structures called M pro-teins of which there are now more than 120
antigenically distinct molecules that
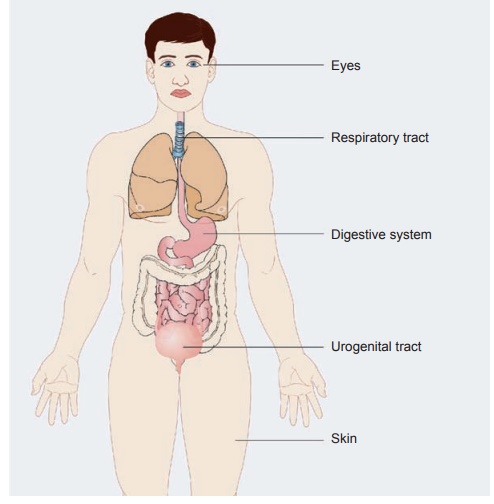
Figure 4.1 Schematic
drawing of the body showing the various organs and systems that offer nonspecific resistance to infection.
inhibit direct phagocytosis, mainly by preventing
deposition of complement on the organism. Another example is the pneumococcal
polysaccharide capsule of which there are thirty to forty distinct
polysaccharides. Another approach (taken by both group A streptococci and
staphy-lococci) is the release of potent extracel-lular toxins, which kill
phagocytes with the formation of pus. An intriguing bac-terium, Mycobacterium tuberculosis, can be
ingested by phagocytes but resists intra-cellular killing, often persisting for
years in the macrophage.
Over the past decade, our knowledge of how we sense
the microbial world (innate or adaptive) has fundamentally changed. It has been
known for decades that microbial products such as lipopoly
Although the structures of many dif-ferent
pathogenic microbial compounds have been extensively studied, the molec-ular
basis of their recognition by the cells of the innate immune system remained
elusive. Charles Janeway first developed the concept of microbial structures
forming pathogen-associated molecular patterns (PAMPs), which would be
recognized by pattern recognition receptors. The discovery of a family of toll
receptors (toll refers to the toll gene of Drosophila,
initially identified as an essential receptor controlling dorsoventral
polarization during embryonic development of the fly lar-vae) in species as
diverse as Drosophila fly and humans
and the recognition of their
From these humble beginnings, the field of
mammalian toll-like receptors (TLR) quickly evolved as a crucial sys-tem for
alerting the host to the presence of numerous infectious agents. Infectious
microbes display certain molecular pat-terns that are necessary for microbial
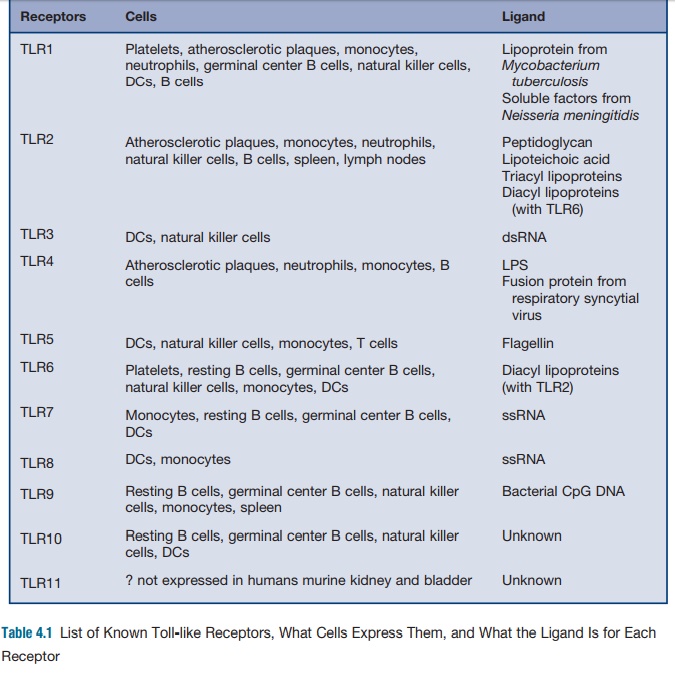
virulence (see Table 4.1). Many of these molecular patterns such as
lipopolysaccha-ride in the outer membranes of gram-nega-tive bacteria seem to
be particularly potent activators of mammalian cells. The mam-malian receptors
responsible for recogni-tion of PAMPs are called pattern recognition receptors.
The failure of the immune system to
recognize a pathogen’s PAMP could lead to a delay or blunting of the immune
response, resulting in unchecked invasion by the microbe.
Perhaps the best example of an incom-plete
recognition by the TLR system is a gram-negative bacterial infection in the
C3H/HeJ mouse. As few as two colony-forming units of Salmonella typhimurium can kill this mouse. Further exploration of
this extraordinary virulence revealed that the mouse harbored a point mutation
(P712H) in the TLR4, which results in defective sig-nal transduction in
response to LPS and a heightened susceptibility to gram-negative infections.
The family of TLRs is a highly special-ized system
that can identify a number of microbial and endogenous ligands and acti-vate
the immune system to respond. The body must be able to respond differently to
various challenges. Thus, the specificity in the immune response via TLRs is
becoming increasingly complex. Currently, probes of the TLR family have been
described, and it is expected that many more receptors will be discovered in
the future.
Related Topics