Chapter: Basic & Clinical Pharmacology : Nitric Oxide
Nitric Oxide Synthesis, Signaling Mechanisms, & Inactivation
NITRIC OXIDE
SYNTHESIS, SIGNALING MECHANISMS, & INACTIVATION
Synthesis
NO,
written as NO• to indicate an unpaired electron in its chemical
structure, or simply NO, is a highly reactive signaling molecule that is made
by any of three closely related NO synthase (NOS, EC 1.14.13.49) isoenzymes,
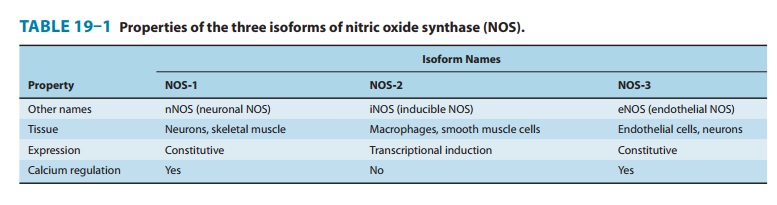
each of which is encoded by a separate gene and named for the initial cell type
from which it was isolated (Table 19–1). These enzymes, neuronal NOS (nNOS or
NOS-1), macrophage or inducible NOS (iNOS or NOS-2), and endothelial NOS (eNOS
or NOS-3), despite their names, are each expressed in a wide variety of cell
types, often with an over-lapping distribution. These isoforms generate NO from
the amino acid L-arginine in an O2- and NADPH-dependent reaction
(Figure 19–1). This enzymatic reaction involves enzyme-bound cofactors,
including heme, tetrahydrobiopterin, and flavin adenine dinucleotide (FAD). In
the case of nNOS and eNOS, NO synthe-sis is triggered by agents and processes
that increase cytosolic cal-cium concentrations. Cytosolic calcium forms
complexes with calmodulin, an abundant calcium-binding protein, which then
binds and activates eNOS and nNOS. On the other hand, iNOS is not regulated by
calcium, but is constitutively active. In mac-rophages and several other cell
types, inflammatory mediators induce the transcriptional activation of the iNOS
gene, resulting in accumulation of iNOS and increased synthesis of NO.
Signaling Mechanisms
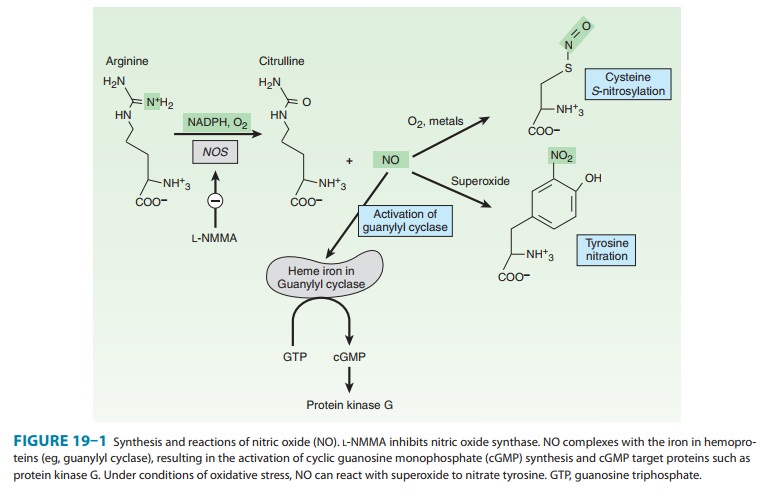
NO mediates
its effects by
covalent modification of
proteins.There are three major targets of NO (Figure 19–1):
1. Metalloproteins—NO interacts with metals, especially ironin heme. The major target of NO is soluble guanylyl cyclase (sGC), heme-containing enzyme that generates cyclic guanosine monophosphate (cGMP) from guanosine triphosphate (GTP).
NO binds to the heme in sGC, resulting in enzyme activation
and elevation in intracellular cGMP levels. cGMP activates protein kinase G
(PKG), which phosphorylates specific proteins. In blood vessels, NO-dependent
elevations in cGMP and PKG activity result in the phosphorylation of proteins that
lead to reduced cytosolic calcium levels and subsequently reduced contraction
of vascular smooth muscle. Interaction of NO with other metallo-proteins
mediates some of the cytotoxic effects of NO associated with NO overproduction,
eg, by activated macrophages. For example, NO inhibits metalloproteins involved
in cellular respira-tion, such as the citric acid cycle enzyme aconitase and
the electron transport chain protein cytochrome oxidase. Inhibition of
heme-containing cytochrome P450 enzymes by NO is a major patho-genic mechanism
in inflammatory liver disease.
2. Thiols—NO reacts with thiols (compounds containing the–SH group) to form nitrosothiols. In proteins, the thiol moiety is found in the amino acid cysteine. This posttranslational modification, termed S-nitrosylation or S-nitrosation, requires either met-als or O2 to catalyze the formation of the nitrosothiol adduct. S-nitrosylation is highly specific, with only certain cysteine resi-dues in proteins becoming S-nitrosylated. S-nitrosylation can alter the function, stability, or localization of target proteins.
Although the physiologic roles of protein nitrosylation are not fully established,
major targets of S-nitrosylation are
H-ras, a regulator of cell proliferation that is activated by S-nitrosylation, and the metabolic
enzyme glyceraldehyde-3-phosphate dehydrogenase, which is inhibited when it is S-nitrosylated. Denitrosylation of
proteins is poorly understood but may involve enzymes, such as thioredoxin, or
chemical reduction by intracellular reducing agents such as glutathione, an
abundant intracellular sulfhydryl-containing compound. Glutathione can also be S-nitrosylated under physiologic conditions
to generate S-nitrosoglutathione. S-nitrosoglutathione may serve as an
endogenous stabilized formof NO or as a carrier of NO. Vascular glutathione is
decreased in diabetes mellitus and atherosclerosis, and the resulting
deficiency of S-nitrosoglutathione
may account for the increased incidence of cardiovascular complications in
these conditions.
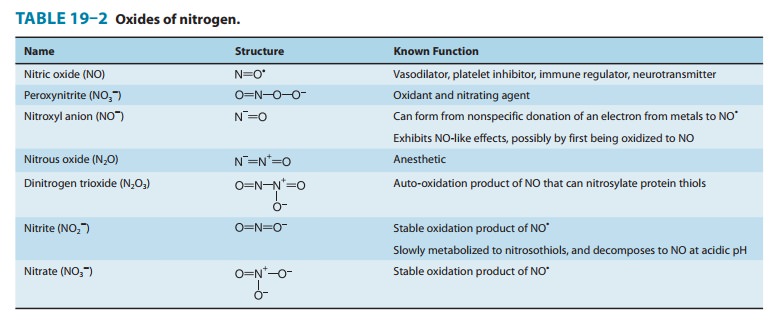
3. Tyrosine nitration—NO undergoes both
oxidative andreductive reactions, resulting in a variety of oxides of nitrogen
that can nitrosylate thiols and nitrate tyrosines (described below) or
arestable oxidation products (Table 19–2). NO reacts very efficiently with
superoxide to form peroxynitrite (ONOO–), a highly reactive oxidant
that leads to DNA damage, nitration of tyrosine, andoxidation of cysteine to disulfides
or to various sulfur oxides (SOx). Several cellular enzymes
synthesize superoxide, and the activity of these enzymes, as well as NO
synthesis, is increased in numerous inflammatory and degenerative diseases,
resulting in an increase in peroxynitrite levels. Numerous proteins are
susceptible to peroxynitrite-catalyzed tyrosine nitration, and this
irreversible modification can be associated with either activation or
inhibition of protein function. Detection of tyrosine nitration in tissue is
often used as a marker of oxidative stress and tissue damage, although a direct
causal role of tyrosine nitration in the pathogen-esis of any disease has not
been definitively established. Peroxynitrite-mediated protein modification is
mitigated by intra-cellular levels of glutathione, which can protect against
tissuedamage by scavenging peroxynitrite. Factors that regulate the
biosynthesis and decomposition of glutathione may have impor-tant consequences
on the toxicity of NO.
Inactivation
NO
is highly labile due to its rapid reaction with metals, O2, and
reactive oxygen species. NO can react with heme and hemopro-teins, including
oxyhemoglobin, which oxidizes NO to nitrate. The reaction of NO with hemoglobin
may also lead to S-nitrosylation of
hemoglobin, resulting in transport of NOthroughout the vasculature. NO is also
inactivated by reaction with O2 to form nitrogen dioxide. NO reacts
with superoxide, which results in the formation of the highly reactive
oxidizing spe-cies, peroxynitrite. Scavengers of superoxide anion such as
super-oxide dismutase may protect NO, enhancing its potency and prolonging its
duration of action.
Related Topics