Chapter: Pharmaceutical Drug Analysis: Polarimetry
Nephelometric Assay of Pharmaceutical Substances
NEPHELOMETRIC ASSAY
Nephelometric assay may be employed for the determination
of sulphate (SO 2–) and phosphate (P 43–) ions
quite efficiently. These two estimations shall be discussed below in an
elaborated manner :
1. Assay of Sulphate Ion (SO42–)
Theory :
From actual experience it has
been observed that it is always difficult to reproduce the turbid-ity of a
dilute barium-sulphate-suspension. Hence, it is very important to adopt the
underlying experimental procedure very closely and rigidly so as to obtain
reasonably good results, namely :
(i) The rate of
formation (velocity) of the precipitation along with the concentration of the
reactants should be monitored and controlled by the addition of pure solid
barium chloride having a definite grain size,
(ii) The rate
at which barium chloride undergoes dissolution controls the velocity of the
reaction,
(iii) Both NaCl
and HCl (reagent) are added before the commencement of the precipitation so as
to check the growth of microcrystals of BaSO4,
(iv) An optimum
pH must be maintained that essentially decreases the effect of variable
quantities of the other electrolytes, possibly present in the sample, upon the
size of the suspended BaSO4 particles,
(v) Most
importantly the presence of glycerol-ethanol solution helps to stabilize the
turbidity,
(vi) Each
reaction-vessel must be shaken gently both at the same rate and the same number
of times so as to obtain a uniform particle size (BaSO4),
(vii) The
unknown sample should be treated exactly in an identical manner (as the
standard solution), and
(viii) The
time-gap between the time of precipitation and the time of measurement (of
turbidity) should always be kept constant.
Materials Required
(i)
Standard Sulphate Solution : 1.814 g of K2SO4
(dry) is dissolved in DW and diluted to 1 L in a graduated flask :
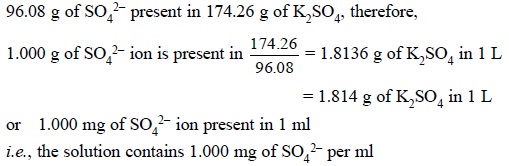
(ii) Sodium Chloride-Hydrochloric Acid Reagent :
60 g of NaCl is dissolved in 200-ml of DW, add to it 5 ml of concentrated HCl
(AR) and dilute to 250-ml with DW,
(iii) Barium Chloride : The BaCl2
crystals that pass through the 20 mesh sieve and retained by the 39 mesh sieve
are only used,
(iv) Glycerol-Ethanol-Solution : Prepared by
dissolving pure glycerol in absolute ethanol (1 : 2).
Procedure
·
Transfer 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5 and 4.0 ml of
the standard potassium sulphate solution from a burette into each separate
100-ml volumetric flask and number them from 1 to 8,
·
To each flask (1 to 8) pipette out 10 ml of the NaCl-HCl
reagent and 20 ml of the glycerol-ethanol solution, and dilute to 100 ml mark
with DW,
·
Weigh and add 0.3 g of sieved BaCl2 to each
flask (1 to 8) stopper them, and shake for exactly one minute by inverting
flask once in one second (All BaCl2 must dissolve),
·
Permit each flask to stand for 2-3 minutes and read out
the turbidity in the nephelometer,
Caution : Avoid any tiny
air-bubbles sticking to the inner walls of the matched test-tubes.
·
By employing the most-concentrated, K2SO4
solution, as standard, and by the help of the sensitivity control, adjust the
micro-ammeter reading to 100-divisions,
·
A ‘Blank’
solution is prepared by adopting the above operations sequentially, but without
the addi-tion of the K2SO4 solution,
·
Insert the Blank solution in the nephelometer and adjust
to zero reading of the scale by the aid of zero-control-knob,
·
Check the reading of the most-turbid-solution, and adjust
any deviation from 100 by means of the sensitivity control,
·
Repeat the measurements with the remaining standard
sulphate solution and plot the nephelometer reading VS the SO42– ion content per ml,
·
‘Unknown Solution’—Determine the SO42–
ion content of an unknown solution, for instance : 0.4 mg per ml, by means of
the standard-calibration-curve.
2. Assay of Phosphate Ion (PO43–)
Theory :
The underlying principle for
the assay of PO43–
ion by nephelometry is the formation of
strychnine-molybdophosphate complex (I).
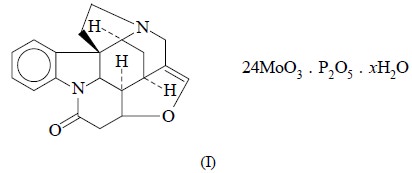
The turbidity thus obtained is white in appearance and
consists of very fine particles of the above complex. Extra care must be taken
for not agitating the precipitate so
as to avoid agglomeration of the same quickly. Likewise, temperature variation
should also be avoided as far as possible because the precipitate is somewhat
sensitive.
Materials Required
·
Standard Phosphate Solution : 1.721 g of KH2PO4,
previously dried at 110 °C, is dissolved in
1 L of DW in a 1000-ml volumetric flask and make up the volume with DW upto
the mark.
The resulting diluted solution contains 0.01 mg P2O5
ml–1.
·
Molybdate-Strychnine Reagent
Solution ‘A’ : (Acid Molybdate Solution) :
Weigh 30 g of molbdenum trioxide (Mo2O3) in a 500-ml conical flask, add to it 10 g of Na2CO3
and 200 ml of DW. Boil the contents of the flask until a clear solution is
achieved. Filter the hot solution, add 200 ml of 5 M. H2SO4,
allow to cool and dilute to 500 ml with DW.
Solution ‘B’
(Strychnine-Sulphate Solution) : Weigh 1.6 g strychnine sulphate in 100 ml of DW.
Warm it gently, cool and dilute to 500 ml with DW.
Molybdate-strychnine reagent is prepared by dissolving
solution-B shaking the resulting mixture vig-orously. The bluish-white
precipitate thus obtained is filtered through What man No : 42 filter paper and
the resulting clear solution may be used within 20 hours.
Note : (i) Strychnine must be handled with gloves on as it is a very toxic
alkaloloidal substance and under no condition it should be ingested,
(ii) Molybdate-strychnine reagent is always prepared afresh by
mixing solution-B to solution-A, because the addition of the acid-molybdate
solution to the strychnine-sulphate solution gives a precipitate after 24
hours, and
(iii) Solutions A and B can be stored indefinitely.
·
Saturated Sodium Sulphate
Solution : A
saturated aqueous solution of sodium sulphate is prepared at 50 °C, cooled to room temperature and filtered before
use.
·
Sulphuric Acid (1 M) : 27.0 ml of concentrated H2SO4 is diluted to 500 ml in a graduated
flask.
Procedure
·
Transfer accurately 1.0, 2.0, 4.0, 6.0, 8.0, and 10.0 ml
of the standard phosphate solution with a burette into each 100 ml volumetric
flasks.
·
18 ml of 1 M . H2SO4 is added to
each flask, followed by 16 ml of saturated sodium sulphate solution, and
diluted to 95 ml with DW.
·
Add 2.0 ml of the molybdate-strychnine reagent to the
resulting solution and make up the volume to 100 ml.
·
The contents of the flask is mixed by gently inverting it
a number of times, but without shaking vigorously.
·
Keep the flasks aside for at least 20 minutes so as to
allow the turbidities to develop before making the measurements.
·
A ‘blank’ solution is prepared by performing the above
operations sequentially, but without the addition of the phosphate solution.
·
By employing the most concentrated solution as the
initial standard, adjust the microammeter reading to 100 divisions.
·
Place the ‘blank’ solution into the matched test-tube of
the nephelometer and adjust the reading to zero.
·
Check the reading of the most turbid solution, and adjust
any deviation from 100 by the help of the sensitivity control.
·
Repeat the measurements with the remaining standard
phosphate solution and plot the nephelometer reading VS the mg P2O5 per ml.
·
Unknown Solution : Determine the phosphate
content of an unknown solution, for example : containing 0.005 mg P2O5 per ml by the help
of the standard-calibration graph.
Related Topics