Chapter: Essentials of Anatomy and Physiology: Body Temperature and Metabolism
Metabolism
METABOLISM
The term metabolism encompasses all of the reac-tions that take place in the body. Everything that hap-pens within us is part of our metabolism. The reactions of metabolism may be divided into two major cate-gories: anabolism and catabolism.
Anabolism means synthesis or “formation” reac-tions, the bonding together of smaller molecules to form larger ones. The synthesis of hemoglobin by cells of the red bone marrow, synthesis of glycogen by liver cells, and synthesis of fat to be stored in adipose tissue are all examples of anabolism. Such reactions require energy, usually in the form of ATP.
Catabolism means decomposition, the breaking of bonds of larger molecules to form smaller molecules. Cell respiration is a series of catabolic reactions that break down food molecules to carbon dioxide and water. During catabolism, energy is often released and used to synthesize ATP (the heat energy released was discussed in the previous section). The ATP formed during catabolism is then used for energy-requiring anabolic reactions.
Most of our anabolic and catabolic reactions are catalyzed by enzymes. Enzymes are proteins that enable reactions to take place rapidly at body temperature. The body has thousands of enzymes, and each is specific, that is, will catalyze only one type of reaction. As you read the discussions that follow, keep in mind the essential role of enzymes.
CELL RESPIRATION
You are already familiar with the summary reaction of cell respiration,
C6H12O6 + O2 → CO2 + H2O + ATP 1 Heat, (glucose)
the purpose of which is to produce ATP. Glucose con-tains potential energy, and when it is broken down to CO2 and H2O, this energy is released in the forms of ATP and heat. The oxygen that is required comes from breathing, and the CO2 formed is circulated to the lungs to be exhaled. The water formed is called metabolic water, and helps to meet our daily need for water. Energy in the form of heat gives us a body tem-perature, and the ATP formed is used for energy-requiring reactions. Synthesis of ATP means that energy is used to bond a free phosphate molecule to ADP (adenosine diphosphate). ADP and free phos-phates are present in cells after ATP has been broken down for energy-requiring processes.
The breakdown of glucose summarized here is not quite that simple, however, and involves a complex series of reactions. Glucose is taken apart “piece by piece,” with the removal of hydrogens and the split-ting of carbon–carbon bonds. This releases the energy of glucose gradually, so that a significant portion (about 40%) is available to synthesize ATP.
Cell respiration of glucose involves three major stages: glycolysis, the Krebs citric acid cycle, and the cytochrome (or electron) transport system. Although the details of each stage are beyond the scope of this book, we will summarize the most important aspects of each, and then relate to them the use of amino acids and fats for energy. This simple summary is depicted in Fig. 17–3.
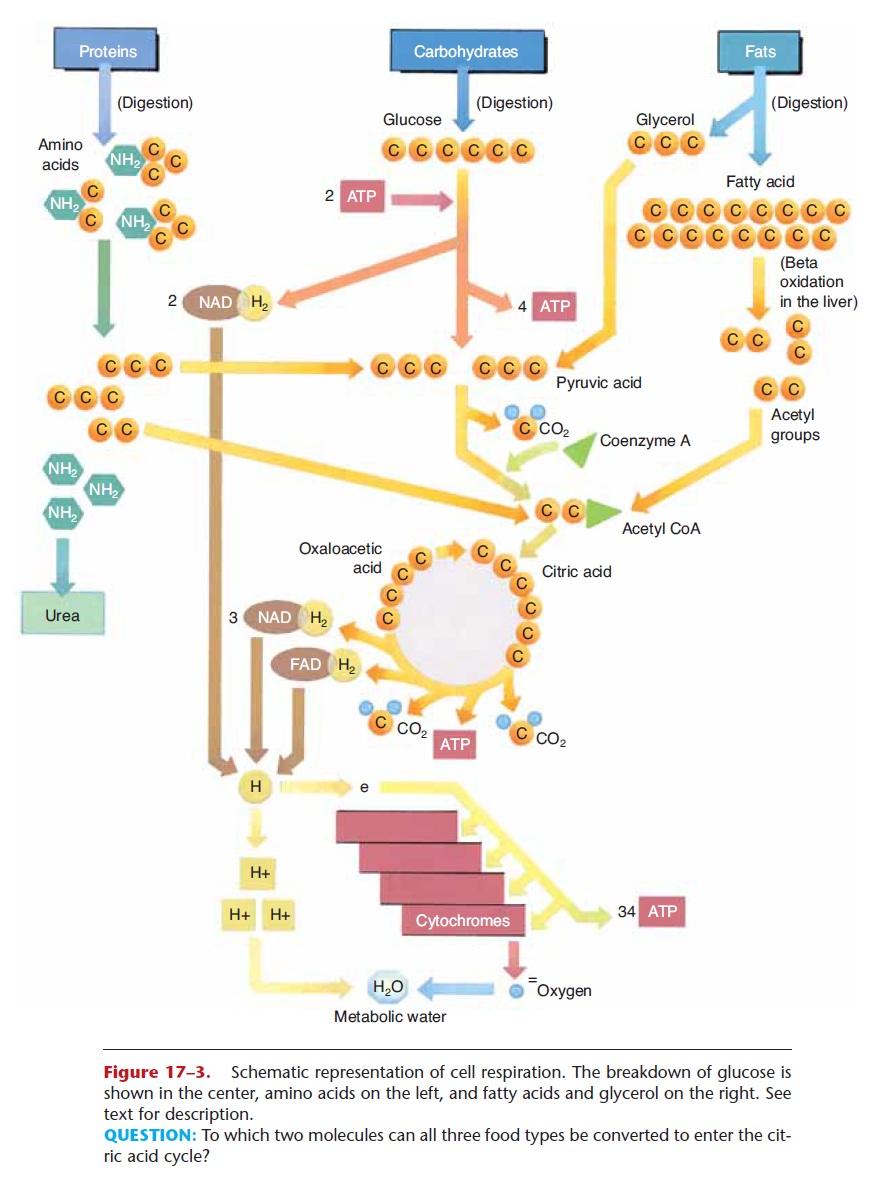
Figure 17–3. Schematic representation of cell respiration. The breakdown of glucose is shown in the center, amino acids on the left, and fatty acids and glycerol on the right. See text for description.
QUESTION: To which two molecules can all three food types be converted to enter the cit-ric acid cycle?
Glycolysis
The enzymes for the reactions of glycolysis are found in the cytoplasm of cells, and oxygen is not required (glycolysis is an anaerobic process). Refer now to Fig. 17–3 as you read the following. In glycolysis, a six-carbon glucose molecule is broken down to two three-carbon molecules of pyruvic acid. Two molecules of ATP are necessary to start the process. The energy they supply is called energy of activation and is neces-sary to make glucose unstable enough to begin to break down. As a result of these reactions, enough energy is released to synthesize four molecules of ATP, for a net gain of two ATP molecules per glucose molecule. Also during glycolysis, two pairs of hydrogens are removed by NAD, a carrier molecule that contains the vitamin niacin. Two NAD molecules thus become 2NADH2, and these attached hydrogen pairs will be transported to the cytochrome transport system (stage 3).
If no oxygen is present in the cell, as may happen in muscle cells during exercise, pyruvic acid is converted to lactic acid, which causes muscle fatigue. If oxygen is present, however, pyruvic acid continues into the next stage, the Krebs citric acid cycle (or, more simply, the Krebs cycle).
Krebs Citric Acid Cycle
The enzymes for the Krebs cycle (or citric acid cycle) are located in the mitochondria of cells. This second stage of cell respiration is aerobic, meaning that oxygen is required. In a series of reactions, a pyru-vic acid molecule is “taken apart,” and its carbons are converted to CO2. The first CO2 molecule is removed by an enzyme that contains the vitamin thiamine. This leaves a two-carbon molecule called an acetyl group, which combines with a molecule called coen-zyme A to form acetyl coenzyme A (acetyl CoA). As acetyl CoA continues in the Krebs cycle, two more carbons are removed as CO2, and more pairs of hydro-gens are picked up by NAD and FAD (another carrier molecule that contains the vitamin riboflavin). NADH2 and FADH2 will carry their hydrogens to the cytochrome transport system.
During the Krebs cycle, a small amount of energy is released, enough to synthesize one molecule of ATP (two per glucose). Notice also that a four-carbon mol-ecule (oxaloacetic acid) is regenerated after the forma-tion of CO2. This molecule will react with the next acetyl CoA, which is what makes the Krebs cycle truly a self-perpetuating cycle. The results of the stages of cell respiration are listed in Table 17–3. Before you continue, you may wish to look at that table to see just where the process has gotten thus far.
Cytochrome Transport System
Cytochromes are proteins that contain either iron or copper and are found in the mitochondria of cells. The pairs of hydrogens that were once part of glucose are brought to the cytochromes by the carrier mole-cules NAD and FAD. Each hydrogen atom is then split into its proton (H+ ion) and its electron. The electrons of the hydrogens are passed from one cytochrome to the next, and finally to oxygen. The reactions of the electrons with the cytochromes release most of the energy that was contained in the glucose molecule, enough to synthesize 34 molecules of ATP. As you can see, most of the ATP produced in cell respiration comes from this third stage.
Finally, and very importantly, each oxygen atom that has gained two electrons (from the cytochromes) reacts with two of the H+ ions (protons) to form water. The formation of metabolic water contributes to the necessary intracellular fluid, and also prevents acido-sis. If H+ ions accumulated, they would rapidly lower the pH of the cell. This does not happen, however, because the H+ ions react with oxygen to form water, and a decrease in pH is prevented.
The summary of the three stages of cell respiration in Table 17–3 also includes the vitamins and minerals that are essential for this process. An important over-all concept is the relationship between eating and breathing. Eating provides us with a potential energy source (often glucose) and with necessary vitamins and minerals. However, to release the energy from food, we must breathe. This iswhy we breathe. The oxygen we inhale is essential for the completion of cell respi-ration, and the CO2 produced is exhaled.
Proteins and Fats as Energy Sources
Although glucose is the preferred energy source for cells, proteins and fats also contain potential energy and are alternative energy sources in certain situations.
As you know, proteins are made of the smaller mol-ecules called amino acids, and the primary use for the amino acids we obtain from food is the synthesis of
Excess amino acids, however, those not needed immediately for protein synthesis, may be used for energy production. In the liver, excess amino acids are deaminated, that is, the amino group (NH2) is removed. The remaining portion is converted to a molecule that will fit into the Krebs cycle. For exam-ple, a deaminated amino acid may be changed to a three-carbon pyruvic acid or to a two-carbon acetyl group. When these molecules enter the Krebs cycle, the results are just the same as if they had come from glucose. This is diagrammed in Fig. 17–3.
Fats are made of glycerol and fatty acids, which are the end products of fat digestion. These molecules may also be changed to ones that will take part in the Krebs cycle, and the reactions that change them usually take place in the liver. Glycerol is a three-carbon molecule that can be converted to the three-carbon pyruvic acid, which enters the Krebs cycle. In the process of beta-oxidation, the long carbon chains of fatty acids are split into two-carbon acetyl groups, which enter a later step in the Krebs cycle (see Fig. 17–3).
Both amino acids and fatty acids may be converted by the liver to ketones, which are two- or four-carbon molecules such as acetone and acetoacetic acid. Although body cells can use ketones in cell respira-tion, they do so slowly. In situations in which fats or amino acids have become the primary energy sources, a state called ketosis may develop; Ketosis. Excess amino acids may also be converted to glucose; this is important to supply the brain when dietary intake of carbohydrates is low. The effects of hormones on the metabolism of food are summarized in Table 17–4.
Energy Available from the Three Nutrient Types
The potential energy in food is measured in units called Calories or kilocalories. A calorie (lowercase “c”) is the amount of energy needed to raise the tem-perature of 1 gram of water 1°C. A kilocalorie or Calorie (capital “C”) is 1000 times that amount of energy.
One gram of carbohydrate yields about 4 kilocalo-ries. A gram of protein also yields about 4 kilocalories. A gram of fat, however, yields 9 kilocalories, and a gram of alcohol yields 7 kilocalories. This is why a diet high in fat is more likely to result in weight gain if the calories are not expended in energy-requiring activities.
You may have noticed that calorie content is part of the nutritional information on food labels. On such labels the term calorieactually means Calorie or kilo-calories but is used for the sake of simplicity.
SYNTHESIS USES OF FOODS
Besides being available for energy production, each of the three food types is used in anabolic reactions to synthesize necessary materials for cells and tissues.
A simple summary of these reactions is shown in Fig. 17–4. The three food types and their end products of digestion are at the bottom of the picture, and the arrows going upward indicate synthesis and lead to the products formed. You may wish to refer to Fig. 17–4 as you read the next sections.
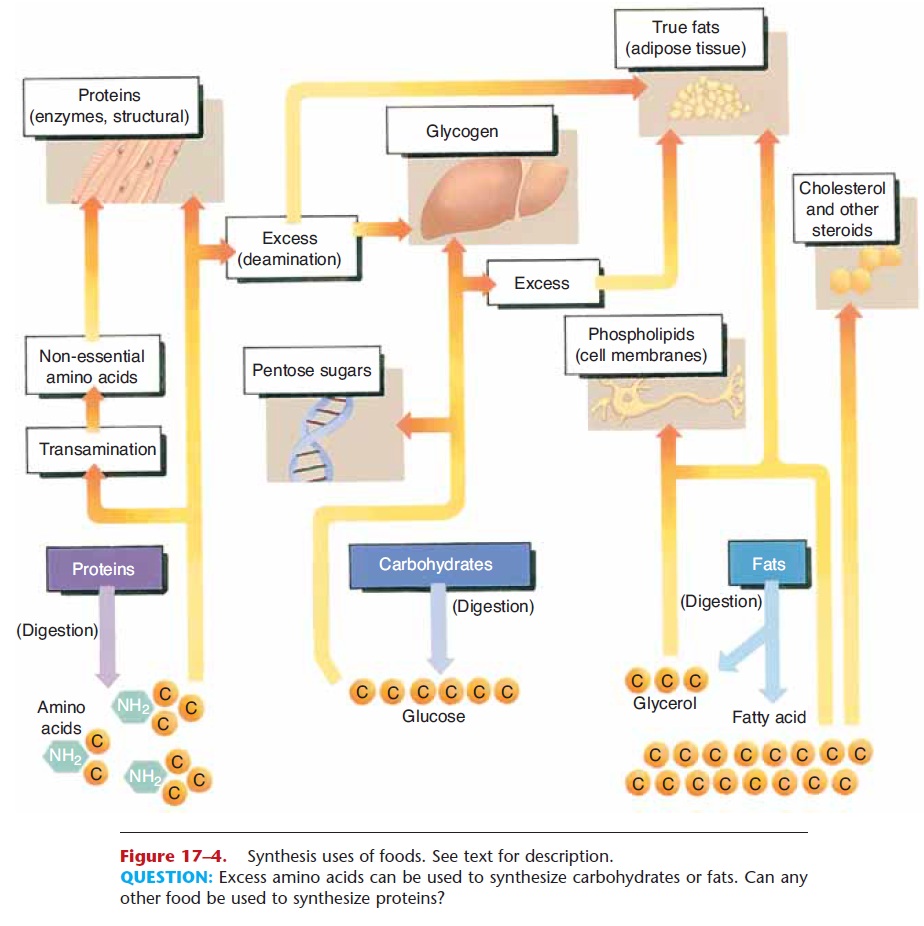
Figure 17–4. Synthesis uses of foods. See text for description.
Glucose
Glucose is the raw material for the synthesis of another important monosaccharide, the pentose sug-ars that are part of nucleic acids. Deoxyribose is the five-carbon sugar found in DNA, and ribose is found
in RNA. This function of glucose is very important, for without the pentose sugars our cells could neither produce new chromosomes for cell division nor carry out the process of protein synthesis.
Any glucose in excess of immediate energy needs or the need for pentose sugars is converted to glycogen in the liver and skeletal muscles. Glycogen is then an energy source during states of hypoglycemia or during exercise. If still more glucose is present, it will be changed to fat and stored in adipose tissue.
Amino Acids
As mentioned previously, the primary uses for amino acids are the synthesis of the non-essential amino acids by the liver and the synthesis of new proteins in all tissues. By way of review, we can mention some proteins with which you are already familiar: keratin and melanin in the epidermis; collagen in the dermis, tendons, and ligaments; myosin, actin, and myoglobin in muscle cells; hemoglobin in RBCs; antibodies pro-duced by WBCs; prothrombin and fibrinogen for clotting; albumin to maintain blood volume; pepsin and amylase for digestion; growth hormone and insulin; and the thousands of enzymes needed to cat-alyze reactions within the body.
The amino acids we obtain from the proteins in our food are used by our cells to synthesize all of these proteins in the amounts needed by the body. Only when the body’s needs for new proteins have been met are amino acids used for energy production. But notice in Fig. 17–4 what happens to excess amino acids; they will be deaminated and converted to simple carbohydrates and contribute to glycogen storage or they may be changed to fat and stored in adipose tissue.
Fatty Acids and Glycerol
The end products of fat digestion that are not needed immediately for energy production may be stored as fat (triglycerides) inadipose tissue. Most adipose tis-sue is found subcutaneously and is potential energy for times when food intake decreases. Notice in Table 17–4 that insulin promotes fat synthesis and storage. One theory of weight gain proposes that a diet high in sugars and starches stimulates the secretion of so much insulin that fat can only be stored, not taken out of storage and used for energy.
Fatty acids and glycerol are also used for the syn-thesis of phospholipids, which are essential compo-nents of all cell membranes. Myelin, for example, is a phospholipid of the membranes of Schwann cells, which form the myelin sheath of peripheral neurons.
The liver can synthesize most of the fatty acids needed by the body. Two exceptions are linoleic acid and linolenic acid, which areessential fatty acids and must be obtained from the diet. Linoleic acid is part of lecithin, which in turn is part of all cell membranes. Vegetable oils are good sources of these essential fatty acids.
When fatty acids are broken down in the process of beta-oxidation, the resulting acetyl groups may also be used for the synthesis ofcholesterol, a steroid. This takes place primarily in the liver, although all cells are capable of synthesizing cholesterol for their cell mem-branes. The liver uses cholesterol to synthesize bile salts for the emulsification of fats in digestion. The steroid hormones are also synthesized from choles-terol. Cortisol and aldosterone are produced by the adrenal cortex, estrogen and progesterone by the ovaries, and testosterone by the testes.
VITAMINS AND MINERALS
Vitamins are organic molecules needed in very small amounts for normal body functioning. Some vitamins are coenzymes; that is, they are necessary for the functioning of certain enzymes. Others are antioxi-dant vitamins, including vitamins C, E, and beta-carotene (a precursor for vitamin A). Antioxidants prevent damage from free radicals, which are mole-cules that contain an unpaired electron and are highly reactive. The reactions of free radicals can damage DNA, cell membranes, and the cell organelles. Free radicals are formed during some normal body reac-tions, but smoking and exposure to pollution will increase their formation. Antioxidant vitamins com-bine with free radicals before they can react with cel-lular components. Plant foods are good sources of these vitamins. Table 17–5 summarizes some impor-tant metabolic and nutritional aspects of the vitamins we need.
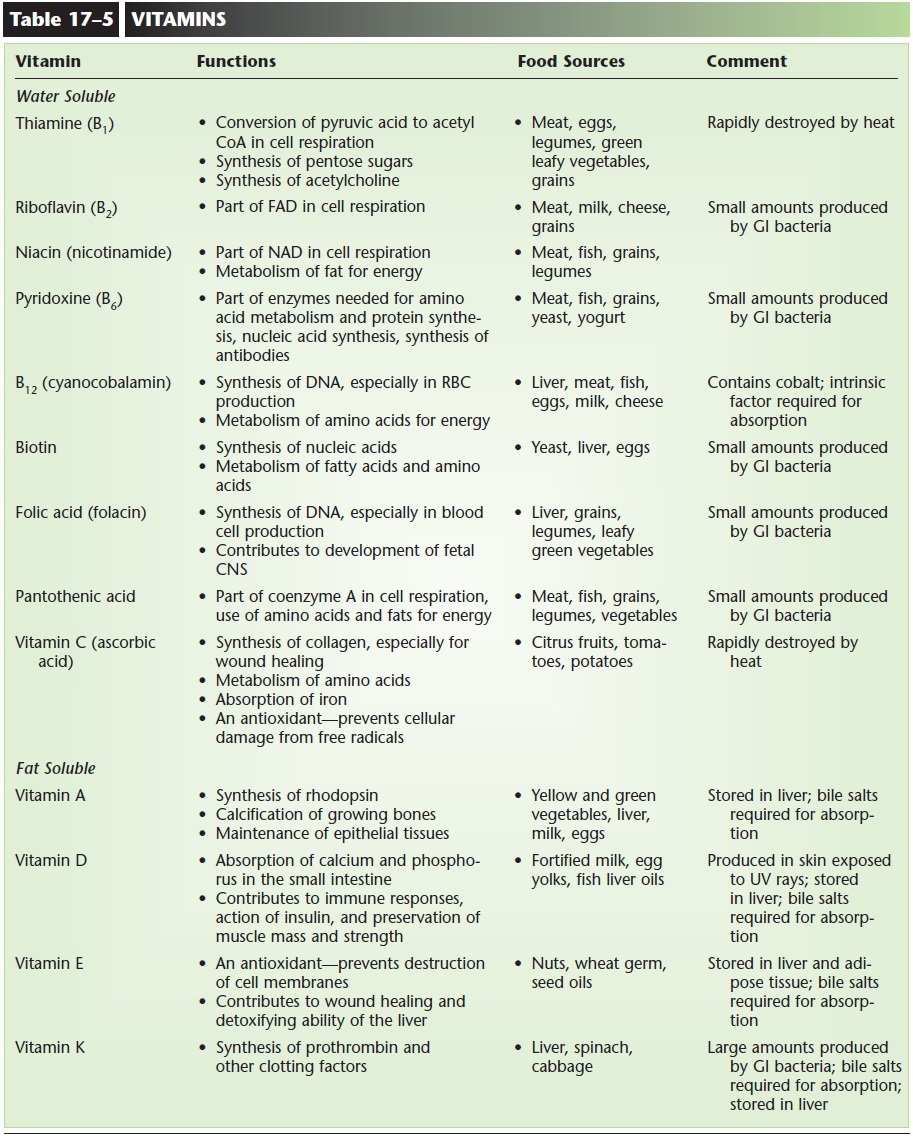
Deficiencies of vitamins often result in disease: vitamin C deficiency and scurvy, for example. Other deficiency diseases that have been known for decades include pellagra (lack of niacin), beri-beri (riboflavin), pernicious anemia (B12), and rickets (D). More recently the importance of folic acid (folacin) for the development of the fetal central nervous sys-tem has been recognized. Adequate folic acid during pregnancy can significantly decrease the chance of spina bifida (open spinal column) and anencephaly (absence of the cerebrum, always fatal) in a fetus. All women should be aware of the need for extra (400 micrograms) folic acid during pregnancy.
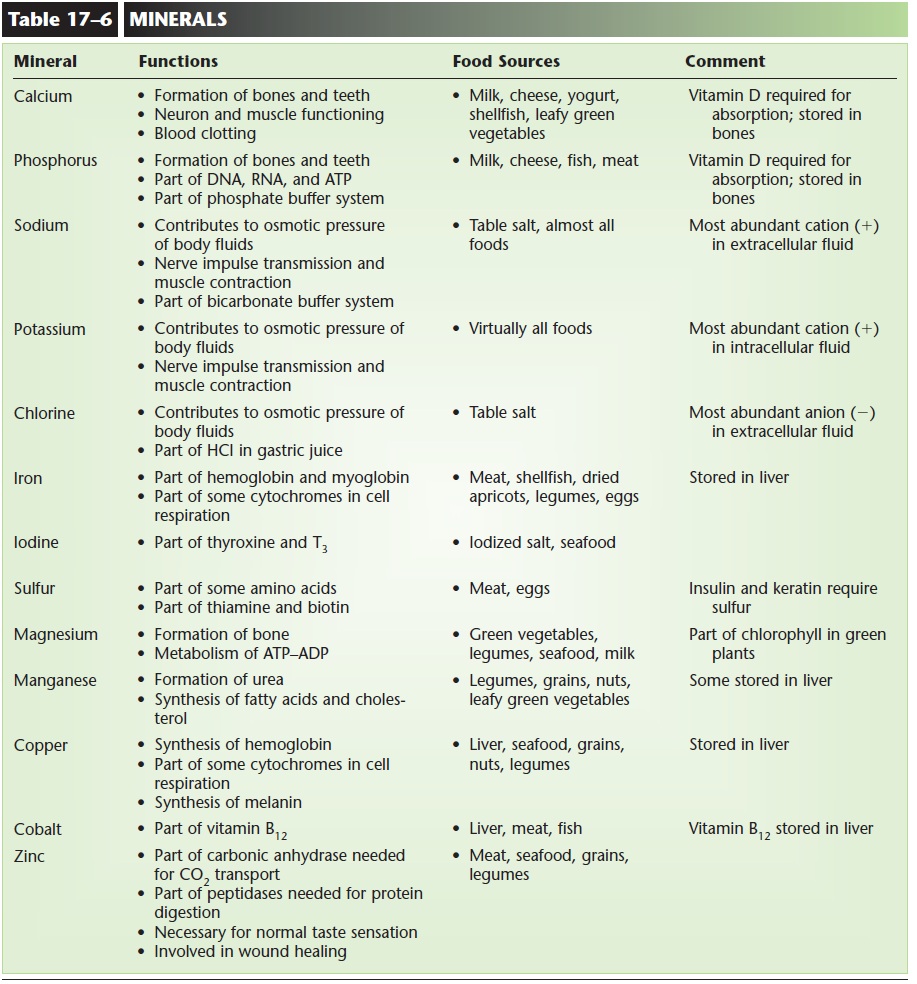
Minerals are simple inorganic chemicals and have a variety of functions, many of which you are already familiar with. Table 17–6 lists some important aspects of minerals.
METABOLIC RATE
Although the term metabolism is used to describe all of the chemical reactions that take place within the body, metabolic rate is usually expressed as an amount of heat production. This is because many body processes that utilize ATP also produce heat. These processes include the contraction of skeletal muscle, the pumping of the heart, and the normal breakdown of cellular components. Therefore, it is possible to quantify heat production as a measure of metabolic activity.
As mentioned previously, the energy available from food is measured in kilocalories (kcal). Kilocalories are also the units used to measure the energy expended by the body. During sleep, for example, energy expended by a 150-pound person is about 60 to 70 kcal per hour. Getting up and preparing breakfast increases energy expenditure to 80 to 90 kcal per hour. For mothers with several small children, this value may be signifi-cantly higher. Clearly, greater activity results in greater energy expenditure.
The energy required for merely living (lying qui-etly in bed) is the basal metabolic rate (BMR).
A number of factors affect the metabolic rate of an active person:
1. Exercise—Contraction of skeletal muscle increases energy expenditure and raises metabolic rate.
2. Age—Metabolic rate is highest in young children and decreases with age. The energy require-ments for growth and the greater heat loss by a smaller body contribute to the higher rate in chil-dren. After growth has stopped, metabolic rate decreases about 2% per decade. If a person becomes less active, the total decrease is almost 5% per decade.
3. Body configuration of adults—Tall, thin people usually have higher metabolic rates than do short, stocky people of the same weight. This is so because the tall, thin person has a larger surface area (pro-portional to weight) through which heat is con-tinuously lost. The metabolic rate, therefore, is slightly higher to compensate for the greater heat loss. The variance of surface-to-weight ratios for different body configurations is illustrated in Fig. 17–5.
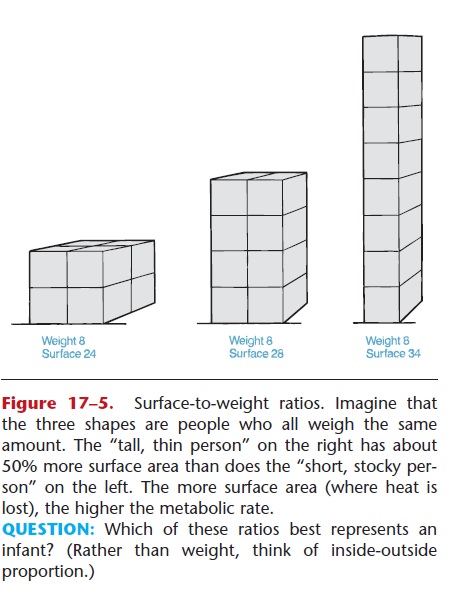
QUESTION: Which of these ratios best represents an infant? (Rather than weight, think of inside-outside proportion.)
4. Sex hormones—Testosterone increases metabolic activity to a greater degree than does estrogen, giv-ing men a slightly higher metabolic rate than women. Also, men tend to have more muscle, an active tissue, whereas women tend to have more fat, a relatively inactive tissue.
5. Sympathetic stimulation—In stress situations, the metabolism of many body cells is increased. Also contributing to this are the hormones epinephrine and norepinephrine. As a result, metabolic rate increases.
6. Decreased food intake—If the intake of food decreases for a prolonged period of time, metabolic rate also begins to decrease. It is as if the body’s metabolism is “slowing down” to conserve what-ever energy sources may still be available.
7. Climate—People who live in cold climates may have metabolic rates 10% to 20% higher than peo-ple who live in tropical regions. This is believed to be due to the variations in the secretion of thyrox-ine, the hormone most responsible for regulation of metabolic rate. In a cold climate, the necessity for greater heat production brings about an increased secretion of thyroxine and a higher meta-bolic rate.
Related Topics