Chapter: Modern Pharmacology with Clinical Applications: Antiprotozoal Drugs
Malaria
MALARIA
Malaria is a parasitic
disease endemic in parts of the world where moisture and warmth permit the
disease vector, mosquitoes of the genus Anopheles,
to exist and multiply. The emergence of both drug-resistant strains of malarial
parasites and insecticide-resistant strains of Anopheles has contributed significantly to the extensive reappearance of this infection. The
annual global inci-dence of malaria is estimated to be approximately 200
million cases, and in tropical Africa alone, malaria is re-sponsible for the
yearly deaths of more than 1 million children younger than 14 years. Malaria
ranks as a lead-ing cause of mortality in the world today.
Most cases of malaria in the
United States result from individuals who have contracted the disease be-fore
they entered this country. It is also possible to con-tract malaria during a
blood transfusion if the trans-fused blood has been taken from a malaria-infected
individual. Additionally, hypodermic needles previously contaminated by blood
containing malarial parasites can be the source of an infection; this has
occurred when needles are shared among drug addicts.
Effective treatment of
malaria depends on early di-agnosis. Since the patient’s symptoms are often
rela-tively nonspecific, it is crucial to examine stained blood smears for the
presence of the parasite. Even this pro-cedure may be inconclusive during the
early stages of the infection, since the levels of parasitemia can be quite
low. Thus, it is important to repeat the blood smear examination several times
if malaria is suspected.
Once the presence of malarial
parasites has been confirmed, it is vital to identify the particular
plas-modial strain involved, since appropriate use of chemotherapy depends on
the particular species re-sponsible for the acute attack. Unfortunately, mixed
in-fections, that is, simultaneous infections with more than one species of
plasmodia, are often observed. If more than a single species is involved,
treatment appropriate for the elimination of all strains must be instituted to
avoid delayed attacks or misinterpretations.
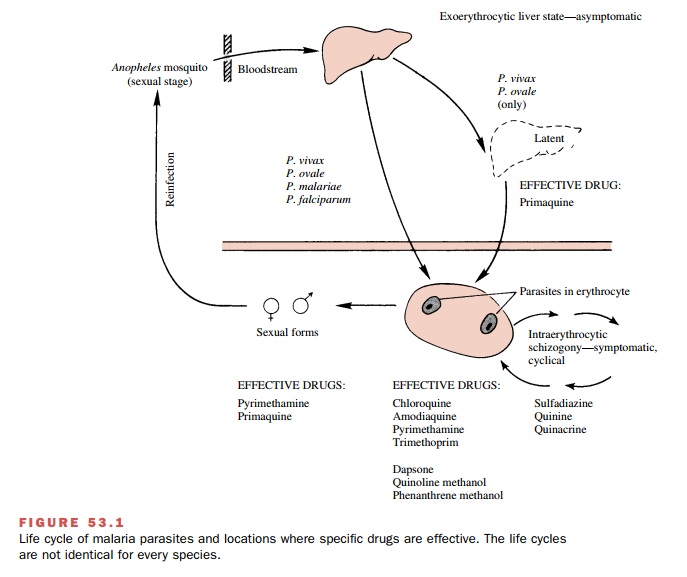
Life Cycle of the Malarial Parasite
The malarial parasite is a
single-cell protozoan (plas-modium). Although more than 100 species of
plasmodia have been identified, only four are capable of infecting humans (Plasmodium malariae, P. ovale, P. vivax,
and P. falciparum); the rest attack a
variety of animal hosts. P. falciparum and P. vivax malaria are the two most common forms.
P. vivax malaria is the most prevalent type of infec-tion and is
characterized by periodic acute attacks of chills and fever, profuse sweating,
enlarged spleen and liver, anemia, abdominal pain, headaches, and lethargy.
Hyperactivity of the reticuloendothelial system and he-molysis are the
principal causes of the enlarged spleen and liver; these effects often result
in anemia, leuko-penia, thrombocytopenia, and hyperbilirubinemia. The cyclical
nature of the acute attacks (48 hours for
P. vivax, P. ovale, and P.
falciparum) is characteristic of malaria
and reflects the relatively synchronous passage of the parasites from one red
blood cell stage in their life cycle to another. If P. vivax malaria is not treated, the symptoms may subside for
several weeks or months and then recur. These relapses are due to a latent
liver form of the parasite (see the following section), which is not present in
P. falciparum strains. Although the
fatal-ity rate of P. vivax malaria is
low, it is an exhausting in-fection and renders the patient more susceptible to
other diseases.
Unchecked P. falciparum malaria is the most
serious and most lethal form of the disease. It is responsible for 90% of the
deaths from malaria. The parasitemia achieved can be quite high and will be
associated with an increased incidence of serious complications (e.g.,
hemolytic anemia, encephalopathy). P.
falciparum malaria produces all of the symptoms listed for P. vivax malaria and in addition can
cause renal failure and pul-monary and cerebral edema. The tissue anoxia
occur-ring in P. falciparum
infections results from the unique sequestering of infected erythrocytes deep
in the capillaries during the last three-fourths of the intraerythro-cytic
cycle.
Members of the genus Plasmodium have a complex life cycle
(Fig. 53.1). A sexual stage occurs within the Anopheles mosquito, while asexual stages take place in the host. Malaria is actually
transmitted from one hu-man to another through the insect vector. Initially, a
fe-male mosquito is infected by biting a human with the disease whose blood
contains male and female gamete forms
of the parasite. Fertilization takes place in the mosquito gut, and after
differentiation and multiplica-tion, the mature sporozoite forms migrate to the insect’s salivary glands. At the
mosquito’s next feeding, the sporozoites are injected into the bloodstream of
an-other human to begin the asexual stages. After a rela-tively brief residence
(less than an hour) in the systemic circulation, the sporozoites invade liver
parenchymal cells, where they divide and develop asexually into multinucleated schizonts. These are the primary
exo-erythrocytic tissue forms of the parasite. When this pri-mary stage of
development is completed (6–12 days), the schizonts will rupture, releasing merozoites into the blood.
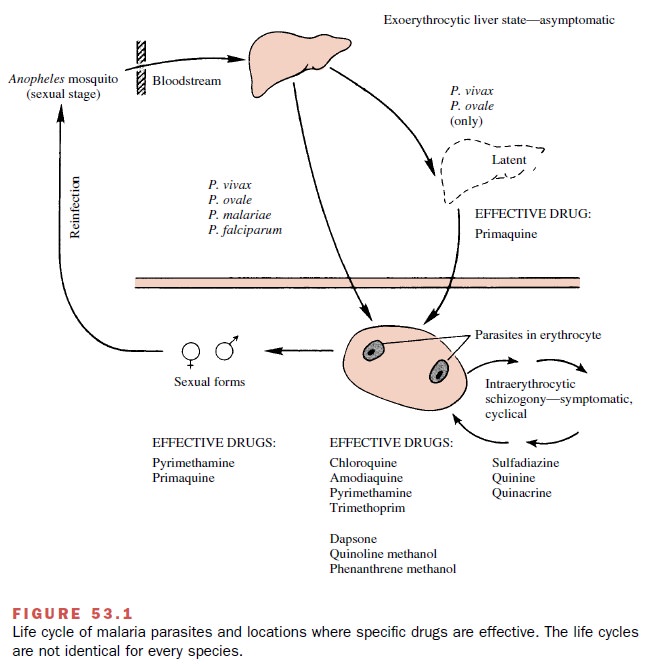
These latter forms
invade host erythrocytes, where they again grow and divide asexually (erythro-cytic schizogony) and become red
cell schizonts. Some of the parasites
differentiate into sexual (male and fe-male) forms, or gametocytes. If the diseased human is bitten by a mosquito at this
time, the gametes will be taken up into the organism’s gut to repeat the sexual
cy-cle. The gametocytes and the exoerythrocytic liver forms of Plasmodium spp. are not associated with
the appearance of clinical symptoms of malaria.
The asexual intraerythrocytic
parasites, that is, those that do not differentiate into gametocytes, also
multiply and grow until they rupture the cells in which they re-side; these new
merozoites are released into the blood-stream. This occurrence not only sets up
the subsequent cyclical red blood cell stages of the cycle but also gives rise
to the symptoms associated with malarial infec-tions. The recurrent chills and
fever are thought to be related to the lysis of erythrocytes and the
accompany-ing release of lytic material and parasite toxins into the
bloodstream. Although the appearance of a cyclic fever is useful for diagnosis,
this symptom may not occur dur-ing the early stages of the infection.
In individuals infected with
either P. vivax or P. ovale,
the exoerythrocytic tissue (e.g., liver) forms can persist after a latent period and give rise to relapses. In P. falciparum and P. malariae malaria, however, there do not appear to be any persistent secondary liver forms. Thus, in
both of these forms of malaria, the physician must contend only with the
asexual erythrocytic forms and the gametes, not with the latent liver forms found
in P. vivax and P. ovale.
Patients who have blood
transfusion malaria are in-fected with the asexual erythrocytic parasites only;
exo-erythrocytic tissue forms apparently do not develop. Plasmodium malariae has been known to produce an infection after transfusion, even when
the blood was ob-tained from a person whose only contact with malaria was 40
years previous to the donation of blood.
Therapeutic Considerations
The main objective in the
clinical management of pa-tients suffering from an acute malaria attack is the prompt elimination of the parasite form
responsible for the symptoms, that is, the asexual erythrocytic form. Drugs
that are particularly effective in this regard are called schizonticidal, or suppressive,
agents. They in-clude such compounds as amodiaquine, chloroguanide,
chloroquine, hydroxychloroquine, pyrimethamine, qui-nine, and tetracycline.
These drugs have the potential (excluding any drug resistance) for effecting a clinical cure; that is, they can reduce the parasitemia to zero. The term radical cure also has been used, and it, in con-trast to clinical
cure, implies the elimination of all para-site forms from the body.
Once the primary therapeutic
objective has been achieved, attention can be focused on such additional
considerations as elimination of the gametocytes and the tissue forms of the
parasite. Success in these areas would help to ensure that relapses do not
occur. Since no latent liver forms are associated with mosquito-in-duced,
drug-sensitive P. falciparum malaria,
administra-tion of chloroquine for up to 3 months after the patient leaves a
malarious area will usually bring about a com-plete or radical cure unless the organism is resistant to
chloroquine.
The emergence of parasites
resistant to chloroquine is an increasingly important problem. Several strains
of chloroquine-resistant P. falciparum
have been identi-fied. This resistance would lead to the reappearance of overt
symptoms of P. falciparum malaria.
P. falciparum malaria may be accompanied by an in-fection caused by one of the
other three plasmodial forms (mixed
infection). As long as all of the parasites are drug sensitive, the
parasitemia can be eliminated. However, it must be remembered that even though P. falciparum
malaria may be ameliorated or eliminated, relapses due to P. vivax
and P. ovale still can occur.
Related Topics