Chapter: Medical Immunology: Cells and Tissues Involved in the Immune Response
Lymphocyte Traffic
LYMPHOCYTE TRAFFIC
The lymphatic and circulatory systems are intimately related (Fig. 2.9), and there is a con-stant traffic of lymphocytes throughout the body, moving from one system to another. Af-ferent lymphatics from interstitial spaces drain into lymph nodes, which “filter” these flu-ids, removing foreign substances. “Cleared” lymph from below the diaphragm and the upper left half of the body drains via efferent lymphatics, emptying into the thoracic duct for subsequent drainage into the left innominate vein.“Cleared” lymph from the right side above the diaphragm drains into the right lymphatic duct with subsequent drainage into the origin of the right innominate vein. The same routes are traveled by lymphocytes stimu-lated in the lymph nodes or peripheral lymphoid tissues, which will eventually reach the systemic circulation.
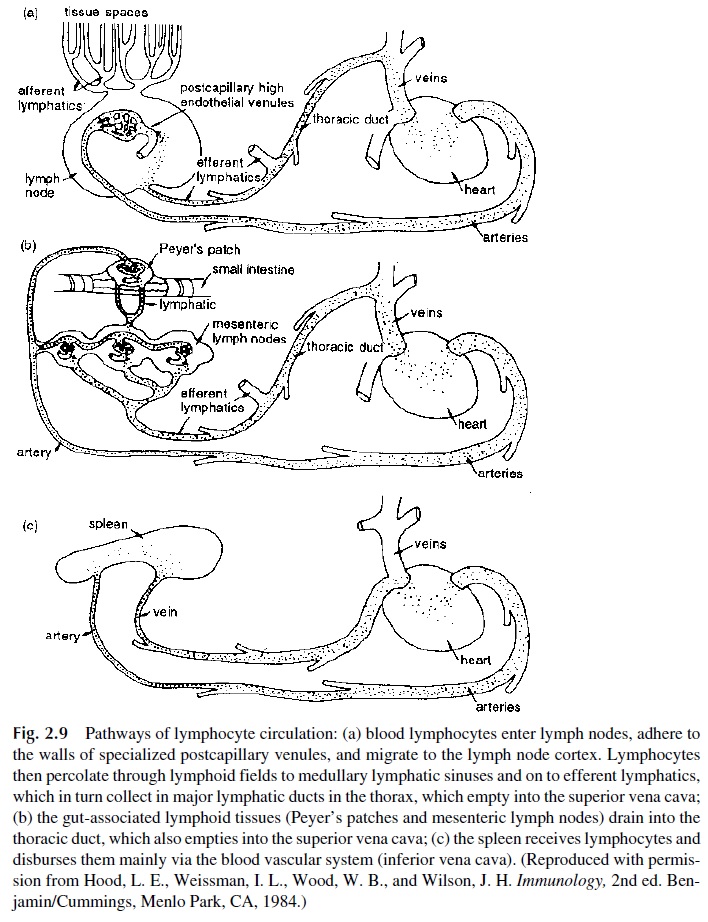
Peripheral blood, in turn, is “filtered” by the spleen and liver, the spleen having or-ganized lymphoid areas while the liver is rich in Kupffer’s cells, which are macrophage-derived phagocytes. Organisms and antigens that enter directly into the systemic circula-tion will be trapped in these two organs, of which the spleen plays the most important role as a lymphoid organ.
A. Lymphocyte Recirculation and Extravascular Migration
One of the most important biological characteristics of B and T lymphocytes is their con-stant recirculation, entering the lymphoid tissues to circulate through the vascular system, enter again the lymphoid tissues, or exit into the interstitial tissues if an inflammatory re-action is taking place.
Lymphocytes circulating in the systemic circulation eventually enter a lymph node, exit the systemic circulation at the level of the high endothelial venules (HEV), leave the lymph node with the efferent lymph, and eventually reenter the systemic circulation.
B lymphocytes of mucosal origin circulate between different segments of the mu-cosal-associated lymphoid tissues, including the GALT, the mammary gland–associated lymphoid tissue, and the lymphoid tissues associated with the respiratory tree and urinary tract.
The crucial step in the traffic of lymphocytes from the systemic circulation to a lym-phoid tissue or to interstitial tissues is the crossing of the endothelial barrier by diapedesis at specific locations. Under physiological conditions, this seems to take place predomi-nantly at the level of the high endothelial venules of lymphoid tissues. These specialized endothelial cells express surface molecules—cell adhesion molecules (CAMs)—which in-teract with ligands, including other cell adhesion molecules, expressed on the membrane of T and B lymphocytes. The interplay between endothelial and lymphocyte CAMs deter-mines the traffic and homing of lymphocytes. Cell adhesion molecules are also upregulated during inflammatory reactions and determine the extravascular migration of lymphocytes and other white blood cells.
B. Cell Adhesion Molecules
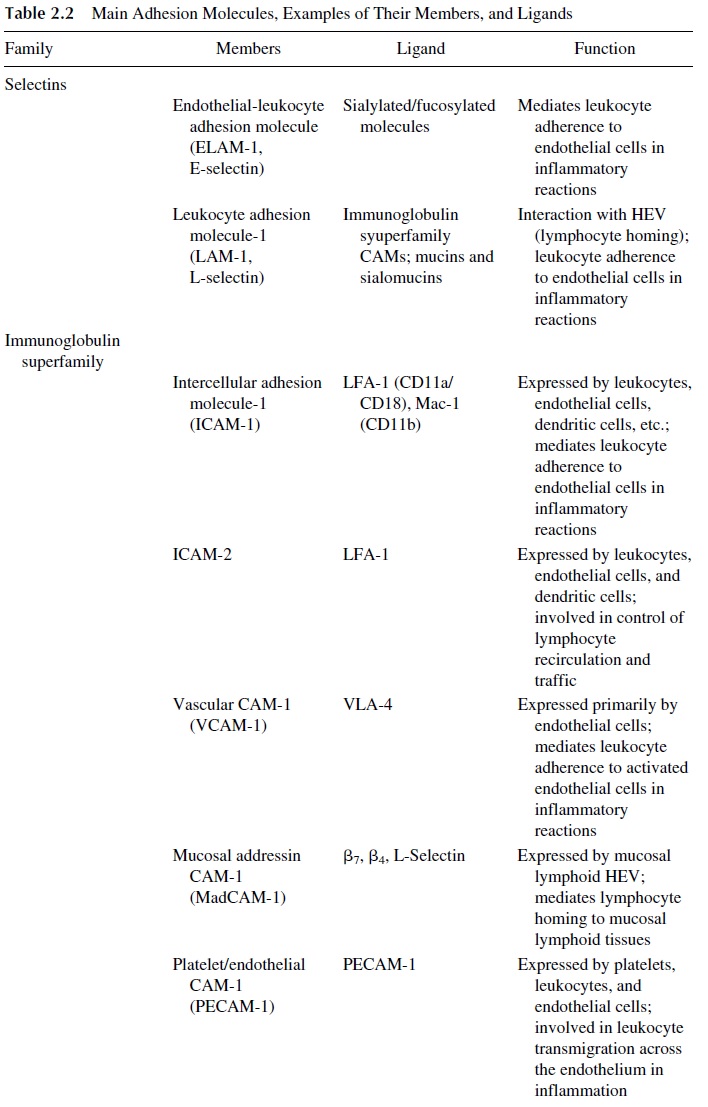
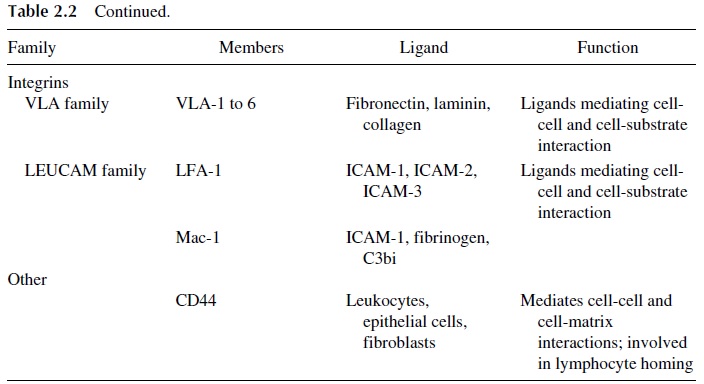
Three main families of cell adhesion molecules have been defined (Table 2.2). The ad-dressins or selectins are expressed on endothelial cells and leukocytes and mediate leuko-cyte adherence to the endothelium. The immunoglobulin superfamily of CAMs includes a variety of molecules expressed by leukocytes, endothelial cells, and other cells. The inte-grins are defined as molecules that interact with the cytoskeleton and tissue matrix com-pounds. The following CAMs have been reported to be involved in lymphocyte traffic and homing:
LAM-1, ICAM-1, and CD44 are primarily involved in controlling lymphocyte traf-fic and homing in peripheral lymphoid tissues.
MadCAM-1 is believed to control lymphocyte homing to the mucosal lymphoid tis-sues.
The interaction between adhesion molecules and their ligands takes place in several stages. First, the cells adhere to endothelial cells at the level of the high endothelium venules (HEV), and the adhering lymphocyte is able then to migrate through endothelial slits into the lymphoid organ parenchyma. Different CAMs and ligands are involved in this sequence of events.
C. Regulation of Lymphocyte Traffic and Homing
The way in which cell adhesion molecules regulate lymphocyte traffic and homing seems to be a result both of differences in the level of their expression and of differences in the nature of the CAM expressed in different segments of the microcirculation. The involve-ment of HEV as the primary site for lymphocyte egress from the systemic circulation is a consequence of the interaction between CD34, a specific CAM expressed in HEV, and L-selectin, expressed by naive T lymphocytes. Because CD34 is predominantly expressed by HEV, the opportunity for cell adhesion and extravascular migration is considerably higher in HEV than on segments of the venous circulation covered by flat endothelium.
It is known that the lymphocyte constitution of lymphoid organs is variable (Table 2.1). T lymphocytes predominate in the lymph nodes, but B lymphocytes and IgA-produc-ing plasma cells predominate in the Peyer’s patches and the GALT in general. This differ-ential homing is believed to be the result of the expression of specific addressins such as MadCAM-1 on the HEV of the perimucosal lymphoid tissues, which are specifically rec-ognized by the B cells and plasma cells resident in those tissues. Most B lymphocytes rec-ognize specifically the GALT-associated HEV and do not interact with the lymph node–as-sociated HEV, while most naive T lymphocytes recognize both the lymph node–associated HEV and the GALT-associated HEV.
The differentiation of T-dependent and B-dependent areas in lymphoid tissues is a poorly understood aspect of lymphocyte “homing.” It appears likely that the distribution of T and B lymphocytes is determined by their interaction with nonlymphoid cells. For exam-ple, the interaction between interdigitating cells and T lymphocytes may determine the pre-dominant location of T lymphocytes in the lymph node paracortical areas and periarteriolar sheets of the spleen, while the interaction of B lymphocytes with follicular dendritic cells may determine the organization of lymphoid follicles in the lymph node, spleen, and GALT.
The modulation of CAM at different states of cell activation explains changing pat-terns in lymphocyte recirculation seen during immune responses. Immediately after anti-gen stimulation, the recirculating lymphocyte appears to transiently lose its capacity to re-circulate. This loss of recirculating ability is associated with a tendency to self-aggregate (perhaps explaining why antigen-stimulated lymphocytes are trapped at the site of maximal antigen density), due to the upregulation of CAMs involved in lymphocyte-lymphocyte and lymphocyte–accessory cell interactions.
After the antigenic stimulus ceases, a population of memory T lymphocytes carrying distinctive membrane proteins can be identified. This population seems to have a different recirculation pattern than that of the naive T lymphocyte, leaving the intravascular com-partment at sites other than the HEV and reaching the lymph nodes via the lymphatic cir-culation. This difference in migration seems to result from the downregulation of the CAMs, which mediate the interaction with HEV selectins, and upregulation of other CAMs, which interact with selectins located in other areas of the vascular tree.
B lymphocytes also change their recirculation patterns after antigenic stimulation. Most B cells will differentiate into plasma cells after stimulation, and this differentiation is associated with marked changes in the antigenic composition of the cell membrane. Con-sequently, the plasma cell precursors (plasmablasts) exit the germinal centers and move into the medullary cords and, eventually, to the bone marrow, where most of the antibody production in humans takes place. Another B-cell subpopulation—the memory B cells— retain B cell markers and reenter the circulation to migrate back to specific territories of the lymphoid tissues.
All memory lymphocytes, T or B, appear to home preferentially in on the type of lymphoid tissue where the original antigen encounter took place, i.e., a lymphocyte that recognizes an antigen in a peripheral lymph node will recirculate to the same or another pe-ripheral lymph node, while a lymphocyte that is stimulated at the GALT level will recircu-late to the GALT. Memory B lymphocytes remain in the germinal centers, while memory T lymphocytes home in on T-cell areas.
Inflammatory and immune reactions often lead to the release of mediators that up-regulate the expression of CAM in venules or in other segments of the microvasculature near the area where the reaction is taking place. This results in a sequence of events that is mediated by different sets of CAMs and their respective ligands.
First the leukocytes slow down and start rolling along the endothelial surface. This stage is mediated primarily by selectins. Next, leukocytes adhere to endothelial cells ex-pressing integrins such as VLA and CAMs of the immunoglobulin superfamily, such as ICAM and VCAM. Finally, the adherent leukocytes squeeze between two adjoining en-dothelial cells and move to the extravascular space.
The end result of this process is an increase in leukocyte migration to specific areas where those cells are needed to eliminate some type of noxious stimulus or to initiate an immune response. As a corollary, there is great interest in developing compounds able to block upregulated CAMs to be used as anti-inflammatory agents.
Related Topics