Chapter: Modern Analytical Chemistry: The Language of Analytical Chemistry
Language of Analytical Chemistry: Protocols
Protocols
Earlier we noted
that a protocol is a set of stringent written
guidelines, specifying an exact
procedure that must
be followed if results are
to be accepted by the
agency specifying the protocol.
Besides all the considerations taken into account
when de- signing the procedure, a protocol also contains very explicit instructions regarding internal and external quality assurance and quality control
(QA/QC) procedures.10 Internal QA/QC
includes steps taken
to ensure that the analytical work in a given
laboratory is both accurate and precise. External
QA/QC usually involves
a process in which
the laboratory is certified by an external
agency.
As an example, we will briefly
outline some of the requirements in the Envi- ronmental Protection Agency’s Contract
Laboratory Program (CLP)
protocol for the analysis of trace metals
in aqueous samples
by graphite furnace
atomic ab- sorption spectrophotometry. The CLP protocol
(Figure 3.7) calls for daily stan-
dardization with a reagent blank
and three standards, one of which
is at the labo- ratory’s contract
required detection limit.
The resulting calibration curve is then verified by analyzing initial
calibration verification (ICV) and initial
calibration blank (ICB) samples. The reported
concentration of the ICV sample must fall within
±10% of the
expected concentration. If the concentration falls outside this limit, the analysis must be stopped
and the problem
identified and corrected be- fore continuing.
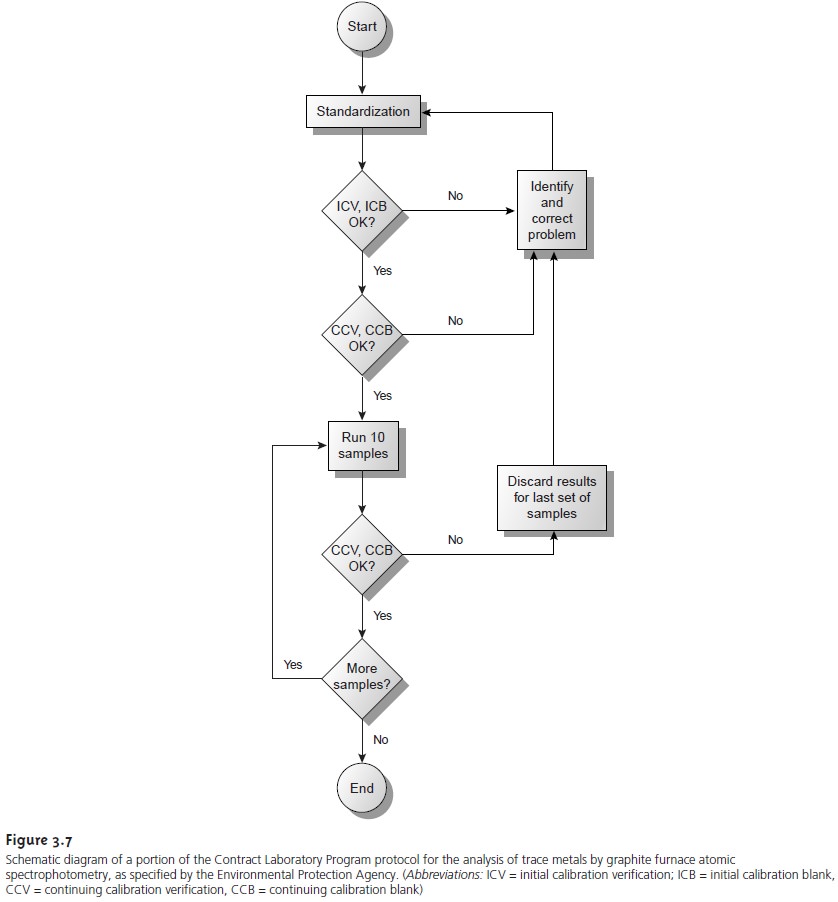
After a successful analysis of the
ICV and ICB
samples, standardization is rever-
ified by analyzing a continuing calibration verification (CCV)
sample and a contin-
uing calibration blank
(CCB). Results for
the CCV also
must be within
±10% of the expected concentration. Again, if the concentration of the CCV falls outside
the es- tablished limits,
the analysis must
be stopped, the
problem identified and
corrected, and the system standardized as described earlier.
The CCV and the CCB are ana- lyzed before the first
and after the last sample,
and after every
set of ten samples.
Whenever the CCV or the CCB is unacceptable, the results for the most recent set of
ten samples are discarded, the system is standardized, and the samples
are reana- lyzed. By following this protocol, every result is bound by successful checks on the standardization. Although
not shown in Figure 3.7, the CLP also contains
detailed instructions regarding the
analysis of duplicate or split samples
and the use
of spike testing for accuracy.
Related Topics