Chapter: Modern Analytical Chemistry: The Language of Analytical Chemistry
Classifying Analytical Techniques
Classifying
Analytical Techniques
Analyzing a sample
generates a chemical or physical signal whose
magnitude is pro- portional to the amount
of analyte in the sample.
The signal may be anything
we can measure; common
examples are mass,
volume, and absorbance. For our pur- poses it is convenient to divide analytical techniques into two general classes
based on whether this signal is proportional to an absolute
amount of analyte
or a relative amount of analyte.

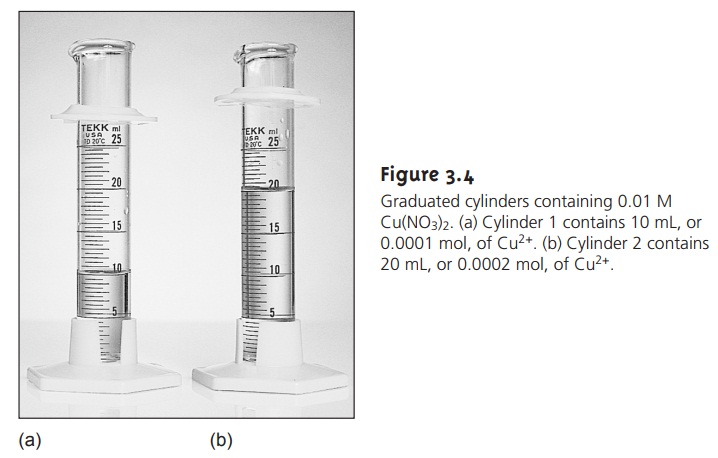
Consider two graduated
cylinders, each containing 0.01 M Cu(NO3)2 (Figure 3.4). Cylinder 1 contains 10 mL, or 0.0001 mol, of Cu2+;
cylinder 2 contains 20 mL, or 0.0002
mol, of Cu2+.
If a technique responds to the absolute
amount of analyte in the sample,
then the signal
due to the analyte, SA, can be expressed as
SA = knA 3.1
where nA is the moles
or grams of analyte in the sample,
and k is a proportionality
constant. Since cylinder 2 contains twice
as many moles
of Cu2+
as cylinder 1, an-
alyzing the contents of cylinder
2 gives a signal that is twice
that of cylinder
1.
A second class of analytical techniques are those that respond
to the relative amount of analyte; thus
SA
= kCA ..........3.2
where CA is the concentration of analyte in the sample.
Since the solutions
in both cylinders have the same concentration of Cu2+, their
analysis yields identical signals.
Techniques responding to the absolute
amount of analyte
are called total analysis
techniques. Historically, most early analytical methods used total
analysis techniques, hence they are often
referred to as “classical” techniques. Mass, volume, and charge are the most common signals for total analysis
techniques, and the cor-
responding techniques are gravimetry , titrimetry , and coulometry . With a few exceptions, the signal in a total
analysis tech- nique results
from one or more chemical
reactions involving the analyte. These re-
actions may involve any combination of precipitation, acid–base, complexation, or
redox chemistry. The
stoichiometry of each
reaction, however, must
be known tosolve equation 3.1 for the moles of
analyte.
Techniques, such as spectroscopy , potentiometry , and voltammetry , in which
the signal is proportional to the relative amount of analyte in a sample are called
concentration techniques. Since
most concentration techniques rely
on measuring an optical or electrical signal,
they also are known as “instrumental” techniques. For a concentration technique, the rela- tionship between
the signal and
the analyte is a theoretical function that depends
on experimental conditions and the instrumentation used to measure
the signal. For this
reason the value
of k in equation 3.2 must be determined experimentally.
Related Topics