Chapter: Modern Analytical Chemistry: The Language of Analytical Chemistry
Language of Analytical Chemistry: Developing the Procedure
Developing the
Procedure
After selecting a method, it is necessary to develop a procedure that will accomplish the goals of the analysis. In developing the procedure, attention is given to compen-
sating for interferences, selecting and calibrating equipment, standardizing the method, acquiring a representative sample, and validating the method.
Compensating for Interferences
The accuracy of a method
depends on its
selectivity for the
analyte. Even the
best methods, however, may not be free from interferents that contribute to the mea- sured signal. Potential interferents may be present in the sample itself or the reagents used during the analysis. In this section
we will briefly
look at how to mini- mize these two sources
of interference.
In the absence
of an interferent, the total
signal measured during
an analysis, Smeas, is a sum
of the signal
due to the
analyte, and the
signal due to the rea- gents, Sreag
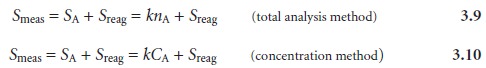
Without an independent determination of Sreag, equation
3.9 or 3.10 cannot be solved for the moles
or concentration of analyte. The contribution of Sreag is deter- mined by measuring the
signal for a reagent or method
blank that does
not contain the sample.
Consider, for example, a procedure in which a 0.1-g sample
is dissolved in 100 mL of solvent. After dissolving the sample, several
reagents are added, and
the signal is measured. The reagent blank is prepared
by omitting the sample and adding the reagents to 100 mL of solvent.
When the sample
is a liquid, or is in solu- tion, an equivalent volume
of an inert solvent is substituted for
the sample. Once Sreag is known, it is easy to correct
Smeas for the reagent’s
contribution to the overall
signal.
Compensating for an interference in the sample’s
matrix is more difficult. If the
identity and concentration of the interferent are known, then it can be added
to the reagent blank. In most
analyses, however, the identity or concentration of matrix interferents is not
known, and their
contribution to Smeas is not included in Sreag. In- stead, the signal
from the interferent is included as an additional term
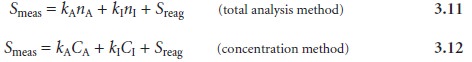
Solving either equation
3.11 or 3.12 for the amount of analyte can be accomplished by separating the analyte
and interferent before the analysis,
thus eliminating the term for the interferent.
Alternatively, equations 3.11 or 3.12 can be solved for the amounts
of both the analyte and the interferent. To do so, however, we must obtain
two independent values for Smeas. Using a concentration method as an example, gives
two equations
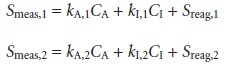
that can be solved
simultaneously for CA and CI. This treatment is general. The composition of a solution
with a total of n analytes and interferents can be deter- mined by measuring n independent signals, and solving
n
independent simultane-
ous equations of the general
form of equation
3.11 or 3.12.

Calibration and Standardization
Analytical chemists make a distinction between calibration and standardization.9 Calibration ensures that the equipment or instrument used to measure
the signal is operating correctly by using
a standard known
to produce an exact signal.
Balances, for example, are calibrated using
a standard weight
whose mass can be traced
to the internationally accepted platinum–iridium prototype kilogram.
Standardization is the process of experimentally determining the relation- ship between the signal and the amount of analyte (the value of k in equations 3.1 and 3.2). For a total
analysis method, standardization is usually defined
by the stoichiometry of the chemical
reactions responsible for the signal.
For a con- centration method, however, the relationship between the signal and the ana- lyte’s
concentration is a theoretical function
that cannot be calculated without experimental measurements. To standardize a method, the value of k is deter-
mined by measuring the signal for one or more standards, each containing a known
concentration of analyte.
When several standards with different concen- trations of analyte are used, the result is best viewed
visually by plotting
Smeas versus the
concentration of analyte
in the standards. Such a plot
is known as a
calibration curve.
Sampling
Selecting an appropriate method helps ensure that an analysis is accurate. It does
not guarantee, however,
that the result
of the analysis will be sufficient to solve the problem under investigation or that a proposed answer will be correct. These latter
concerns are addressed by carefully collecting the samples
to be analyzed.
A proper sampling
strategy ensures that samples are representative of the mate- rial from which they are taken.
Biased or nonrepresentative sampling and contami- nation of samples during
or after their
collection are two sources of sampling error that can lead to significant errors.
It is important to realize
that sampling errors
are completely independent of analysis errors.
As a result, sampling errors
cannot be corrected by evaluating a reagent blank.
Validation
Before a procedure can provide useful
analytical information, it is necessary to demonstrate that it is capable
of providing acceptable results. Validation
is an eval- uation of whether the precision and accuracy obtained
by following the procedure
are appropriate for
the problem. In addition, validation ensures that the
written procedure has sufficient detail so that different analysts
or laboratories following the same procedure obtain
comparable results. Ideally, validation uses a standard sam- ple whose composition closely
matches the samples for which the procedure was developed. The comparison of replicate analyses
can be used to evaluate
the proce- dure’s precision and accuracy. Intralaboratory and interlaboratory differences in the procedure also
can be evaluated. In the absence
of appropriate standards, accuracy can be evaluated by comparing results
obtained with a new method
to those ob- tained using a method
of known accuracy.
Related Topics