Chapter: Surgical Pathology Dissection : The Urinary Tract and Male Genital System
Kidney : Surgical Pathology Dissection
Kidney
General Comments
The
gross examination plays an important role in the evaluation of kidney
specimens. Macroscopic features often provide important clues to the underlying
pathologic process. For example, the accurate pathologic staging of renal neoplasms
can usually be accomplished simply by noting relationships between the tumor
and certain anatomic landmarks. As with other tissues, no kidney specimen
should be dissected without prior knowledge of the patient’s clinical history.
This fundamental rule is especially important when handling kidney specimens in
which the evaluation of glomerular disease relies on im-munofluorescence and
ultrastructural analysis. Thus, before processing even the smallest kid-ney
biopsy, determine from the clinical history whether fresh tissue should be
submitted for these special studies.
Biopsies
Electron
microscopy and immunofluorescence studies are central to the diagnostic
evaluation of kidney biopsies for non-neoplastic disease. Even small specimens
must be properly oriented because glomeruli should be included in the tissue
submitted for each of these tests. The goal of orienting kidney biopsies is to
distinguish cortex from medulla. While this distinction is usually quite simple
with the larger wedge biop-sies, the identification of cortex and medulla in
small-needle biopsies often requires the use of a magnifying lens or dissection
microscope. Under magnification, the cortex can be recognized byits red
sunburst pattern corresponding to the vascular tufts of the glomeruli. In
contrast, the straight vessels of the medulla are seen as thin red streaks
coursing through tan/white tissue. From the tips of the cortical end, freeze a
1-mm section for immunofluorescence, and submit an adjacent 1-mm section in
glutaraldehyde for elec-tron microscopy. If multiple cores are received, do the
same for each core. If you cannot confi-dently identify an end with cortex, do
not guess. Rather, submit 1-mm sections from both ends of the core. For larger
wedge biopsies, freeze a full-thickness section of cortex for
immunofluores-cence, and submit 1-mm3 cubes
from the cortex in glutaraldehyde for electron microscopy. Sub-mit the
remainder of the tissue in fixative for routine histologic processing.
Nephrectomies for Neoplastic Disease
The purpose
of evaluating kidneys resected for neoplasms is to determine the type of tumor,
its extent, and the completeness of the resection. This can easily be achieved
if you approach the nephrectomy as if it were made up of three individual
compartments: the kidney, the renal hilum, and the perinephric fat. Describe
each component of the kidney individually and sys-tematically (Table 33-1).
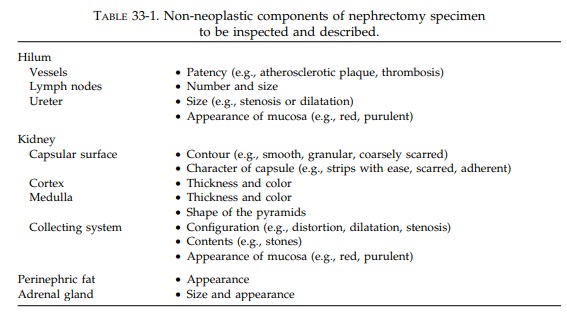
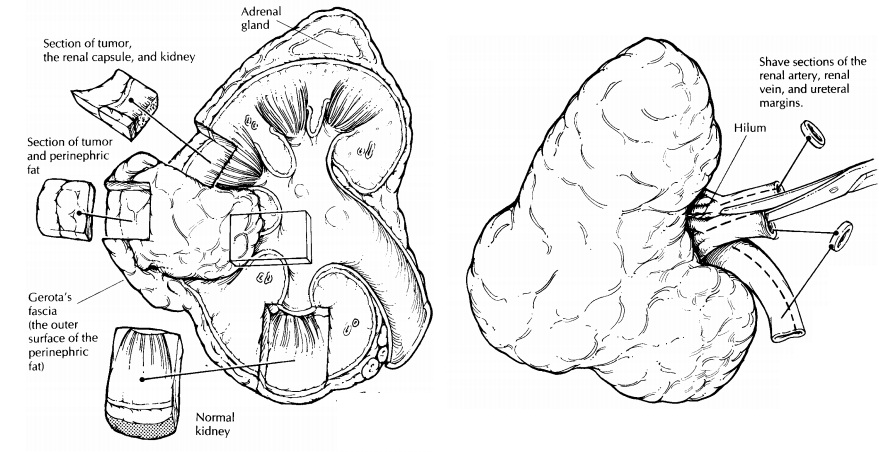
First, weigh and measure the entire specimen. The renal capsule and the perinephric fat should not be stripped from the kidney until after their relationships to the tumor are established. Anat-omically orient the specimen. The ureter will provide a useful landmark: the downward course of the ureter points to the inferior pole of the kidney. By knowing whether the kidney is from the right or left side, you can easily iden-tify its anterior and posterior surfaces.
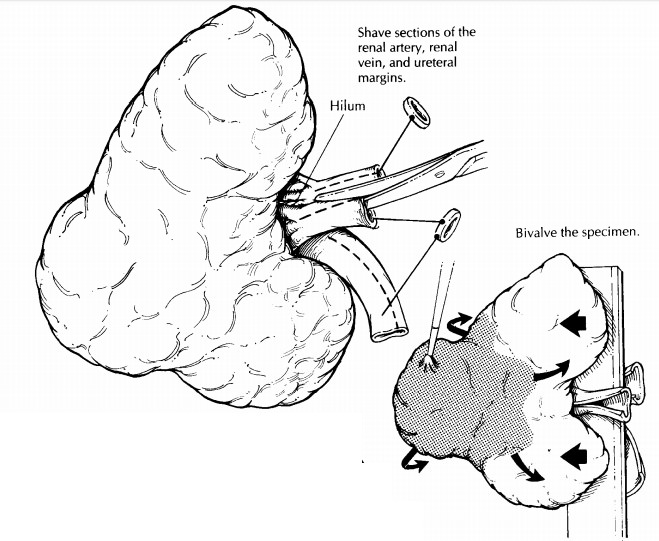
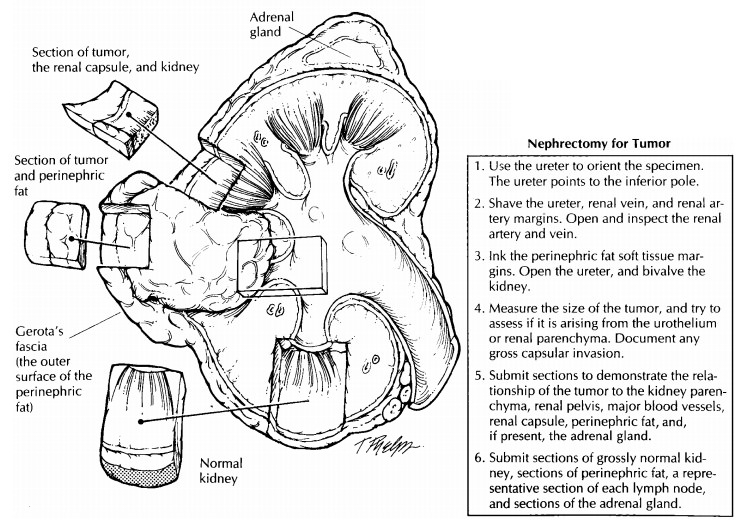
Begin
the dissection at the kidney hilum. Iden-tify the ureter, renal artery, and
renal vein. Shave the margin from each, and then open each with a small pair of
scissors to the point at which they enter the kidney. Look for the presence of
vas-cular invasion, atherosclerosis, and thrombosis. Carefully inspect the mucosa
and wall of the ureter. Is the ureter dilated or strictured? Are any masses
present? Occasionally, lymph nodes will be present in the soft tissues of the
hilum, and these should be individually measured and sam-pled. Other lymph node
groups are usually sub-mitted separately by the surgeon.
Next,
ink the soft tissue margins and direct your attention to the dissection of the
kidney itself. The objective of the initial section is to bivalve the kidney so
that the relationship of the tumor to the kidney can be easily visualized. The
plane of this initial section may vary de-pending on the location of the tumor,
but in gen-eral it will be a sagittal cut that begins at the hilum and exits
laterally through the perinephric fat. It may be helpful to insert probes into
the calyces of the upper and lower poles of the kid-ney to guide the knife
through the renal pelvis. Once the tumor is exposed, obtain fresh tissue for
special studies such as electron microscopy, immunofluorescence, and
cytogenetics, as is in-dicated. Describe the size, shape, color, and
con-sistency of the tumor. Does the tumor appearto be centered in the cortex,
medulla, or pelvis? Measure the distance of the tumor to the nearest margin,
and note its relationship to the peri-nephric fat, renal pelvis, renal vein,
ureter, and adrenal gland. Photograph the bivalved speci-men. At this point you
may choose to complete the dissection of the kidney in its fresh state, or you
may want to submerge the specimen in formalin until it is well fixed.
The
outer surface of the fat represents Gerota’s fascia. Ink this surface where it
overlies the tumor, and submit perpendicular sections to show the relationship
of the tumor to the soft tissue margin. Then carefully peel back the
peri-nephric fat from the kidney, examine the capsular surface, and look for
tumor extension through the renal capsule. Keep in mind that when renal tumors
invade through the capsule, they tend to bulge from the surface of the kidney.
Note any bulges, and document any disruption of the normal contour of the
kidney surface by submit-ting sections that include the tumor, the adja-cent
non-neoplastic kidney, and the overlaying capsule. Make additional sections
through the tumor and surrounding kidney. Look for any satellite tumors.
Submit
at least four sections of tumor (or more if necessary) to demonstrate the
relation-ship of the tumor to the kidney parenchyma, renal pelvis, major blood
vessels, renal capsule, and perinephric fat. Because some renal neo-plasms are
multifocal, section through the re-mainder of the kidney, looking for smaller
tumors. Do not forget to describe the cortex, medulla, and collecting system of
the non-neoplastic kidney. Also, submit one or two sec-tions of the
non-neoplastic kidney for histology. Finally, dissect the fibrofatty tissue
enveloping the kidney, and submit one or two sections of the fat to assess
infiltration by tumor.
Nephrectomy
specimens often will include the adrenal gland. Be sure to look for it in the
superior perinephric fat; if it is present, weigh it, measure it, and submit a
section. Keep in mind that the lymph nodes will be found in the soft tissues at
the kidney hilum. A misdirected search for lymph nodes in the perinephric fat
outside of the hilum will be a waste of your time.
Important Issues to Address in Your Surgical Pathology Report on Nephrectomies for Tumor
· What
procedure was performed, and what structures/organs are present?
· Is a
neoplasm present?
· Where is
the tumor located?
· How
large is the tumor?
· Does the
tumor invade the renal capsule, Gerota’s fascia, major veins, or the adrenal
gland?
· What are
the histologic type and grade of the neoplasm?
· What is
the status of each of the margins (ureter, renal vein, soft tissue)?
· Are
metastases identified? Record the number of nodes involved and the number
examined.
· Does the
non-neoplastic portion of the kidney show any pathology?
Partial Nephrectomies
Occasionally,
you may receive a partial nephrec-tomy—that is, a tumor removed with only a
small portion of surrounding renal parenchyma. Take the same approach as you
would for a total nephrectomy, only remember to sample the renal parenchymal
margins. Ink these margins, and submit perpendicular sections to demon-strate
the distance of the tumor’s edge to the margin. Given the much more limited extent
of these resections, you will often not be able toassess the relationship of
the tumor to the peri-nephric fat, renal vein, ureter, or other impor-tant
structures.
Nephrectomies for Non-neoplastic Disease
Dissection
of the nephrectomy specimen is essen-tially the same for neoplastic and
non-neoplastic diseases. Before beginning the dissection, try to establish from
the patient’s clinical history whether fresh cortical tissue should be taken
for immunofluorescence studies or electron micros-copy. Evaluate the kidney,
the hilum, and the perinephric fat as three separate compartments, using the
guidelines of dissection given earlier. When the specimen shows some
modification (e.g., the absence of a perinephric soft tissue compartment),
simply alter the dissection ac-cordingly. Some pathologists may prefer to
evaluate the texture of the cortical surface by stripping the capsule from the
fresh specimen, and this can be done before the kidney is sec-tioned and fixed.
If
calculi are identified during the dissection, submit some for chemical analysis
if indicated. Because the macroscopic findings often provide crucial clues to
the pathologic process involv-ing the kidney, describe each component of the
kidney individually and systematically (see Table 33-1).
Cystic Kidneys
Sometimes
kidneys are resected for congenital or acquired non-neoplastic cystic disease.
Even for massively enlarged and distorted kidneys, the dissection should follow
the same guidelines given above, only remember to pay particular attention to
the following points: (1) Probe the ureters before opening them to check for
patency.
•
Note the location of the cysts in terms of
their relationship to the cortex and medulla. (3) Docu-ment the size and
contents of the cysts. (4) Last, because cystic kidneys can harbor unsuspected
neoplasms, thoroughly section and inspect the kidney. Submit sections of any
suspicious areas, including solid foci, cysts with thickened walls, and cysts
with a papillary lining.
Related Topics