Chapter: Pharmaceutical Drug Analysis: Karl Fischer Method for Determination of Water
Karl Fischer Method for Determination of Water
KARL FISCHER METHOD FOR DETERMINATION OF WATER
INTRODUCTION
A plethora of chemical compounds for the determination of
small amounts of water present in organic solids, pharmaceutical substances and
organic solvents have been devised over a length of time. But unquestionably
the most important of these is the one proposed by Karl Fischer (1935), which
is considered to be relatively specific for water*. It essentially makes use of
the Karl Fischer reagent which is composed of iodine, sulphur dioxide, pyridine
and methanol.
Note : Both pyridine and
methanol should be anhydrous.
THEORY
Water present in the analyte reacts with the Karl Fischer
reagent in a two-stage process as shown below :
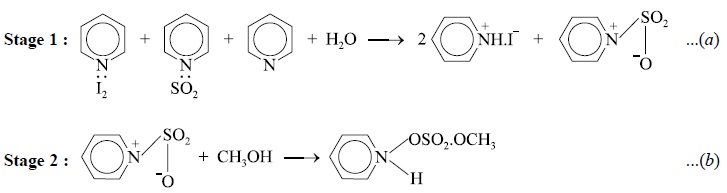
From Eq. (a)
step l, it is obvious that the oxidation of sulphur dioxide takes place by
iodine to yield sulphur trioxide and hydrogen iodide thereby consuming one mole
of water. In other words, each one molecule of iodine disappears against each
molecule of water present in the given sample. It is pertinent to mention here
that in the presence of a large excess of pyridine (C5H5N),
all reactants as well as the resulting products of reaction mostly exist as
complexes as evident from Eqs. (a)
and (b).
Stability of the Reagent : The stability of the original
Karl Fischer reagent initially prepared with an excess of methanol was found to be fairly poor and hence,
evidently needed frequent standardization. However, it was estabtished
subsequently that the stability could be improved significantly by replacing
the methanol by 2-methoxyethanol.
It has been observed that the titer of the Karl Fischer
reagent, which stands at 3.5 mg of water per milliliter of reagent, falls
rapidly upon standing with the passage of time. Hence, the following
precautions must be observed rigidly using the Karl Fischer reagent, namely :
(a) Always
prepare the reagent a day or two before it is to be used,
(b) Great care
must be taken to prevent and check any possible contamination either of the
reagent or the sample by atmospheric moisture,
(c) All
glassware(s) must be thoroughly dried before use,
(d) Standard
solution should be stored out of contact with air, and
(e) Essential
to minimise contact between the atmosphere and the solution during the course
of titration.
End-point Detection : The end-point of the Karl
Fischer titration may be determined quite easily by adopting the electrometric technique employing the dead-stop
end-point method. When a small quantum of e.m.f. is applied across two platinum
electrodes immersed in the reaction mixture, a current shall tend to flow till
free iodine exists, to remove hydrogen and ultimately depolarize the cathode. A
situation will soon arise when practically all the traces of iodine have
reacted completely thereby setting the current to almost zero or very close to
zero or attain the end-point.
Limitations of Karl Fischer
Titration : The
Karl Fischer titration has a number of serious limitations due to possible interferences tantamount to erroneous results,
namely :
(i) Oxidizing agents, for instance :
chromates, Cu(II), Fe(III), Cr2O72–,
peroxides, salts, higher oxides,
Example :
MnO2 + 4C5H5NH+
+ 2I– → Mn2+ + 4C5H5N + I2
+ H2O
(ii) Reducing agents, such as : Sn(II)
salts, sulphides, and S2O32–, and
(iii) Compounds
that have a tendency to form water with the ingredients of the Karl Fischer
reagent, for instance :
(a) basic oxides : e.g., ZnO ;
Example : ZnO + 2C5H5NH+ → Zn2+ + C5H5N + H2O
(b) salts of weak oxy-acids e.g., NaHCO3 ;
Example : NaHCO3 +
C5H5NH+ → Na+ + H2O + CO2 + C5H5N
Note : As H2CO3,
carbonic acid, is very unstable ; hence it splits up to yield a mole each of water
and CO2.
Related Topics