Chapter: Pharmaceutical Drug Analysis: Estimation of Phenols and Related Compounds
Estimation of Phenols and Related Compounds: Assay Methods
ASSAY METHODS
Assay methods based on bromine may be classified under
the following three heads, namely :
(i) Titrations
with 0.1 N Bromine,
(ii) Titrations
with Potassium Bromate, and
(iii)
Titrations with Potassium Iodate.
1. TITRATIONS WITH 0.1 N BROMINE
This involves the preparation of 0.1 N bromine solution
and subsequent standardization with 0.1 N sodium thiosulphate solution. Bromine
solution is also known as Koppeschaar’s Solution in some literature.
1.1. Preparation of 0.1 N Bromine Solution
Materials Required : Potassium bromate : 3.0 g ;
potassium bromide : 15 g.
Procedure : Weigh 3 g of potassium bromate
and 15 g of potassium bromide in a beaker and dissolve with water. Transfer it quantitatively into a 1 litre volumetric
flask and make up the volume with DW.
1.2. Standardization of 0.1 N Bromine with 0.1 N Sodium Thiosulphate Solution
Materials Required : 0.1 N Bromine solution ;
hydrochloric acid ( −~ 11.5 N) : 5 ml ; potassium iodide solution
(10% w/v in water) : 5.0 ml ; 0.1 N sodium thiosulphate ; starch solution.
Procedure : Transfer 25 ml of 0.1 N
bromine solution with the help of a pipette into a 500 ml iodine flask and dilute it with 120 ml of DW.
Add to it 5 ml of hydrochloric acid, moisten the glass-stopper with water and
insert the stopper in the flask. Shake the contents gently. Now, add 5 ml of
potassium iodide solution, again lace the stopper and allow the resulting
mixture to stand for 5 minutes in the dark. Titrate the liberated iodine with
previously standardized 0.1 N sodium thiosulphate solution, adding 3 ml of
freshly prepared starch solution towards the end-point. Each ml of 0.1 N sodium
thiosulphate is equivalent to 0.01598 g of Br2.
1.3. Thymol
Materials Required : Thymol : 0.1 g ; N sodium
hydroxide : 25.0 ml ; dilute hydrochloric acid (10% v/v of HCl) : 20.0 ml ; 0.1
N bromine ; methyl orange solution (0.1% w/v soln. in 20% alcohol).
Procedure : Weigh accurately about 0.1 g
of thymol, transfer to a 250-ml iodine flask and dissolve in 25.0 ml of N sodium hydroxide. Add to
it 20.0 ml of dilute hydrochloric acid and immediatelv titrate with 0.1 N
bromine to within 1 to 2 ml of the calculated end-point. Warm the solution to
about 75°C, add 2 drops of methyl orange solution and continue the titration
gradually while swirling the contents of the flask thor-oughly after each
addition. When the colour of the methyl orange is discharged, add 2 drops of
0.1 N bromine, shake well, add 1 drop of methyl orange solution and shake
vigorously. If the colour of the solution is still red, continue the titration
dropwise and with constant stirring until the red colour of the indicator is
discharged completely. Repeat the alternate addition of 0.1 N bromine and
methyl orange solution until the red colour is discharged after the addition of
the methyl orange solution. Each ml of 0.1 N bromine is equivalent to 0.003755
g of C10H14O.
Calculations : C10H14O + 2Br2 → C10Hl2Br2O + 2HBr
150.22
Since, 1 mole of thymol reacts with 2 mol, 4 equivalent
of bromine under the conditions of the assay, the equivalent weight of thymol
is 37.55 g, 1/4 gramme molecular weight (i.e.,
150.22/4 = 37.55). Therefore, each milliliter of 0.1 N bromine consumed in the
reaction with thymol is equivalent to 0.1 × 0.03755 = 0.003755 g or 0.1 meq. of
thymol (C10H14O).
1.4. Ethacrynic Acid
Theory : Active bromine is liberated
from the standard solution of bromine in an acidic medium (HCl) that subsequently attacks the double
bond present in the side chain of the ethacrynic acid molecule thereby
resulting into the formation of the corresponding dibromo derivative. This
particular reaction takes place quantitatively. Hence, the reactions involved
in this assay may be expressed as follows :
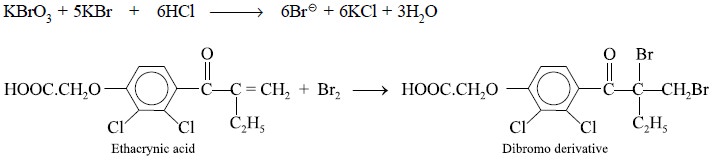
A blank determination is always performed simultaneously
to account for the losses caused by the bromine as well as iodine vapours due
to the interaction of excess bromine on potassium iodide.
Materials Required : Ethacrynic acid : 0.2 g ;
glacial acetic acid : 40.0 ml ; 0.1 N bromine : 20.0 ml ; hydrochloric acid ( −~ 11.5 N) : 3.0 ml ; potassium
iodide solution ; (10% w/v in water) : 20 ml ; 0.1 N sodium thiosulphate ;
starch solution.
Procedure : Weigh accurately about 0.2 g
of ethacrynic acid, dissolve in 40 ml of glacial acetic acid in a 250 ml iodine flask. Add to it 20 ml
of 0.1 N bromine and 30.0 ml of hydrochloric acid, immediately place in
position the moistened stopper to the ffask, mix the contents vigorously and
allow it to stand in a dark place for 60 minutes (to complete the reaction with
bromine). Add to it 100 ml of water and 20 ml of KI Solution and titrate
immediately with 0.1 sodium thiosulphate, employing freshly prepared starch
solution as an indicator towards the end of the titration. Repeat an operation
without the pharmaceutical substance (blank titration) ; thus the difference
between the titrations represents the amount of bromine required by the
ethacrynic acid. Each ml of 0.1 N bromine is equivalent to 0.01516 g of C13H12Cl2O4.
Calculations : From the above equations, we
have :
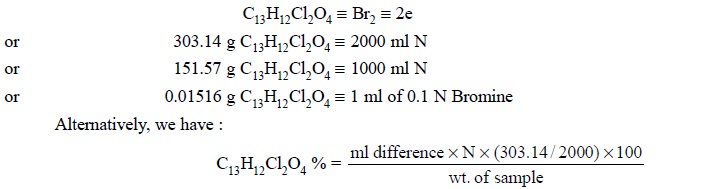
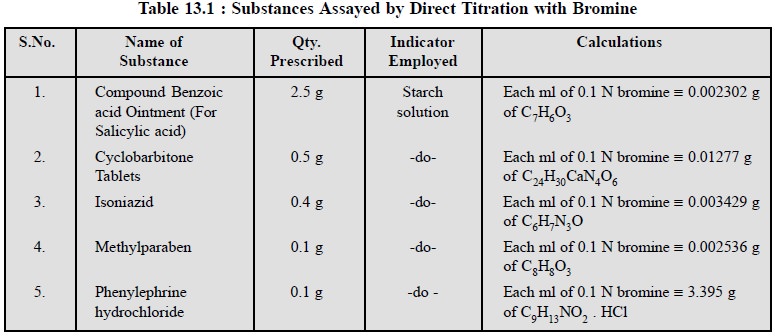
1.5. Cognate Assays
A number of pharmaceutical substances may be determined
quantitatively by titration with bromine as given in Table 13.1.
2. TITRATIONS WITH POTASSIUM BROMATE
Potassium bromate can also be employed as an oxidizing
agent in the assay of a number of pharmaceutical substances, namely :
mephenesin, phenol, and sodium salicylate. This particular method solely
depends upon the formation of iodine monobromide (IBr) in relatively higher
concentration of hydrochloric acid solution.
2.1. Preparation of 0.1 N Potassium Bromate
Theory : Potassium bromate can be
estimated by the addition of potassium iodide and dilute hydrochloric acid. Thus, we have :
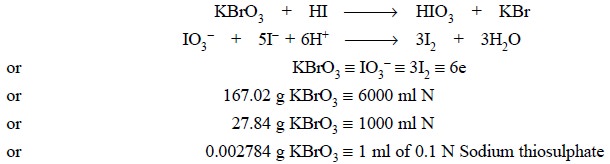
Materials Required : Potassium bromate : 2.784 g.
Procedure : Weigh accurately 2.784 g of
potassium iodide into a beaker and dissolve it in suffcient DW. Transfer the solution quantitatively into a 1 litre volumetric
flask and make up the volume to the mark.
2.2. Standardization of 0.1 N Potassium Bromate Solution with the help of 0.1 N Sodium Thiosulphate
Materials Required : 0.1 N Potassium bromate ;
potassium iodide : 3.0 g ; hydrochloric acid (−~ 11.5 N) : 3.0 ml ; 0.1 N sodium
thiosulphate ; starch solution : 3.0 ml.
Procedure : Transfer an accurately
measured volume of about 30.0 ml of 0.1 N potassium bromate solution into a 250 ml iodine flask. Add to it 3.0 g potassium
iodide, followed by 3.0 ml of potassium iodide, followed by 3.0 ml of
hydrochloric acid. Mix the contents thoroughly and allow it to stand for 5
minutes with its stopper in position. Titrate the liberated iodine with
previously standardized 0.1 N sodium thiosulphate, using 3.0 ml of freshly
prepared starch solution as an indicator at the end-point. Carry out a blank
run using the same quantities of the reagents and incorporate the necessary
corrections, if any. Each ml of 0.1 N sodium thiosulphate is equivalent to
0.002784 g of KBrO3.
2.3. Mephenesin
Theory : Mephenesin undergoes oxidation
with bromine to yield a dibromo derivative as expressed in the following equation :
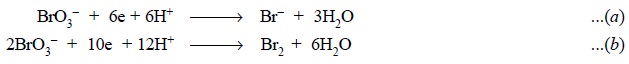
In this instance an excess of potassium bromate is
employed. Therefore, any bromide formed [Eq. (a)] is oxidized to bromine, and the excess bromate and the bromine
are assayed bromometrically. The reduction of bromate to bromine may be
designated as in [Eq. (b)].
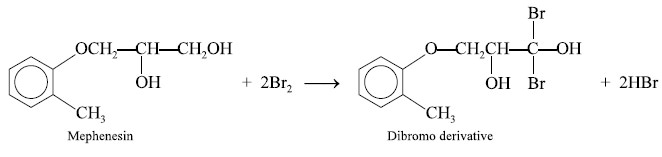
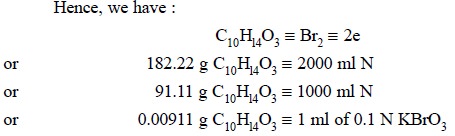
Materials Required : Mephenesin : 0.15 g ; 0.1 N
potassium bromate : 25.0 ml ; potassium bromide powder : 10.0 g ; hydrochloric acid (25% w/v) : 10.0 ml ;
potassium iodide solution (10% w/v in water) : 10.0 ml ; 0.1 N sodium
thiosulphate solution ; starch solution.
Procedure : Weigh accurately 0.15 g of
mephenesin and dissolve in 50 ml of DW into a 250 ml iodine-flask. Add to it
25.0 ml of 0.1 N potassium bromate solution and 10.0 g of powdered potassium
bromide. After the dissolution of KBr, add 10 ml of hydrochloric acid, insert
the moistened stopper, and after 10 seconds add 10 ml of potassium iodide
solution. Titrate with 0.1 N sodium thiosulphate using starch solution as
indicator. Each ml of 0.1 N potassium bromate is equivalent to 0.00911 g of C10Hl4O3.
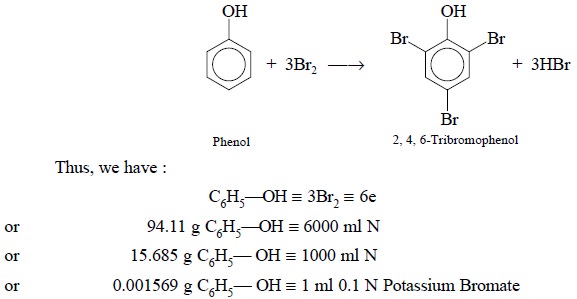
2.4. Phenol
Theory : Phenol interacts with bromine
whereby the former undergoes bromination to yield a water-insoluble 2, 4,
6-tribromophenol. This reaction takes place quantitatively as shown below :
Materials Required : Phenol : 0.5 g ; 0.1 N
potassium bromate : 25.0 ml ; potassium iodide (powdered) : 1.0 g ; dilute
hydrochloric acid (10% w/w of HCl) : 10.0 ml ; potassium iodide (10% w/v in
water) : 10 ml ; chloroform : 10.0 ml ; 0.1 N sodium thiosulphate ; starch
solution.
Procedure : Weigh accurately 0.5 g of
phenol and dissolve in sufficient water to produce 500 ml in a volumetric flask. Mix 25.0 ml of this
solution with 25.0 ml of 0.1 N potassium bromate in a 250 ml iodine flask and
add to it 1 g of powdered KI and 10.0 ml of dilute hydrochloric acid. Moisten
the glass stopper with a few drops of KI solution and place it in position. Set
it aside in a dark place for 20 minutes while shaking the contents frequently
in between. Add to it 10 ml of KI solution, shake the contents thoroughly and
allow it to stand in the dark for a further duration of 5 minutes. Wash the
stopper and neck of the flask carefully with DW, add 10 ml chloroform and
titrate with the liberated iodine with 0.1 N sodium thiosulphate using freshly
prepared starch as an indicator. Carry out a blank titration simultaneously and
incorporate any necessary correction, if required. Each ml of 0.1 N potassium
bromate is equivalent to 0.001569 g of C6H6O.
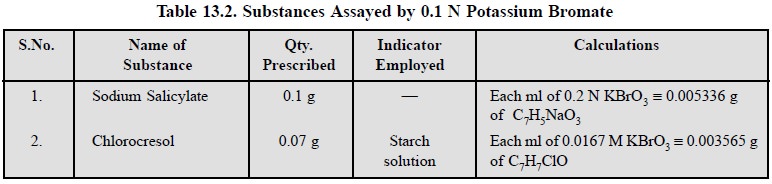
2.5. Cognate Assays
A few other pharmaceutical substances may also be assayed
by titrating with 0.1 N potassium bromate as indicated in Table 13.2.
3. TITRATIONS WITH POTASSIUM IODATE
Potassium iodate is a fairly strong oxidizing agent that
may be used in the assay of a number of pharmaceutical substances, for instance
: benzalkonium chloride, cetrimide, hydralazine hydrochloride, potassium
iodide, phenylhydrazine hydrochloride, semicarbazide hydrochloride and the
like. Under appropriate experimental parameters the iodate reacts
quantitatively with both iodides and iodine. It is, however, interesting to
observe here that the iodate titrations may be carried out effectively in the
presence of saturated organic acids, alcohol and a host of other organic
substances.
The oxidation-reduction methods with potassium iodate
invariably based on the formation of iodine monochloride (ICl) in a medium of
strong hydrochloric acid solution.
3.1. Preparation of 0.05 M Potassium Iodate
Theory : First of all the potassium
iodate is dried to a constant weight at 110°C to make it completely free from moisture and then brought to
room temperature in a desiccator. It is pertinent to mention here that KIO3
is a very stable salt and may be obtained in a very pure form. Therefore, it is
possible to prepare the standard solutions of KIO3 by dissolving the
calculated weight of the salt in water and diluting the same to an approximate
volume.
Since, the normality of iodate solution varies
significantly depending on the nature of the reaction, therefore, in usual
practice standard iodate solutions of known molarity are used.
The reduction of potassium iodate to iodide is usually
not feasible in a direct titrimetric method (unlike the reduction of potassium
bromate to bromide) and hence, has no viable application in the official
procedures
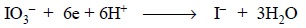 ...........................(a)
...........................(a)
In this type of reaction, 1 mol of KIO3 is 6
equivalent and a 0.05 M solution would be 0.3 N.
In a situation, whereby excess of potassium iodate is
employed, any I– formed [Eq. (a)]
is readily oxidized to iodine, and subsequently the excess iodate and the iodine
are estimated by the iodometric proce-dure. Thus, the reduction of the iodate
to iodine may be expressed as shown below :
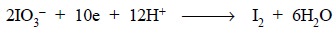 ............................(b)
............................(b)
In such a reaction, 1 mol of iodate is 5 equivalent and a
0.05 M solution would be 0.25 N. This reaction of iodate is never used in the
offcial assay methods.
Interestingly, at higher concentrations of hydrochloric
acid, both the iodide and iodine obtained as reduction products of iodate [Eqs.
(a) and (b)] are quantitatively converted to I+. It forms the basis
of official procedures for iodate titrations.
The iodine produced intially by the reduction of iodate
[Eq. (b)] undergoes solvolysis in a
polar solvent as expressed in the following reaction :

The iodine cation forms iodine monochloride (ICl) in a
medium having sufficiently high concentration of HCl and the latter is
subsequently stabilized by complex ion formation. Thus, we have :
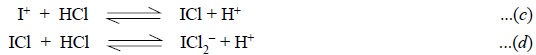
Adding Equations (c)
and (d), we may have :

In actual practice, either carbon tetrachloride or
chloroform is usually added so as to make the end-point distinctly visible.
Iodine is liberated at the initial stages of the titration which renders the
chloroform layer coloured. At that material point when all the reducing agent
under estimation has been duly oxidized, the iodate completes the oxidation of
iodine and iodide to I+, and hence the colour from the chloroform
layer disappears.
In official methods of analysis i.e., the iodine monochloride method, the reduction of KIO3
can be expressed as follows :
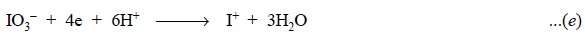
In Eq. (e), 1
mol of KIO3 is 4 equivalent, and a 0.05 solution would be 0.2 N.
Materials Required : Potassium iodate : 10.7 g.
Procedure : Weigh accurately 10.7 g of pure
potassium iodate, previously dried at 110°C to constant weight, in sufficient DW to produce 1 litre in a volumetric flask.
3.2. Benzalkonium Chloride
Materials Required : Benzalkonium chloride : 4.0 g
; chloroform : 60.0 ml ; 0.1 N sodium hydroxide : 10.0 ml ; potassium iodide (5% w/v in water) : 10.0 ml ;
hydrochloric acid ( −~ 11.5 N) : 40.0 ml ; 0.05 M
potassium iodate.
Procedure : Weigh accurately benzalkonium
chloride 4.0 g and dissolve it in sufficient DW to make 100 ml. Pipette 25.0 ml into a separating funnel, add 25 ml of
chloroform, 10 ml of 0.1 N NaOH and 10 ml of potassium iodide solution. Shake
the contents thoroughly, allow to separate and collect the chloroform layer in
another separating funnel. Treat the aqueous layer with 3 further quantities
each of 10 ml of chloroform and discard the chloroform layer. To the aqueous
layer add 40 ml of hydrochloric acid, cool and titrate with 0.05 M potassium
iodate till the solution becomes pale brown in colour. Add 2 ml of chloroform
and continue the titration until the chlorofonn layer becomes colourless.
Titrate a mixture of 29 ml of water, 10 ml of KI solution and 40 ml of
hydrochloric acid with 0.05 M potassium iodate under identical conditions
(Blank Titration). The differences between the titrations represent the amount
of 0.05 M potassium iodate required. Each ml of 0.05 M potassium iodate is
equivalent to 0.0354 g of C22H40ClN.
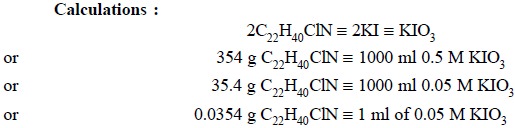
3.3. Potassium Iodide
Theory : The iodine monochloride method
described earlier employing standard potassium iodate is the basis for the official assay of potassium iodide. Vigorous
shaking is a prime requirement, as the end-point is approached in this assay,
because of the fact that both iodine and iodate in different phases attribute a
heterogeneous medium. However, the reaction involving the oxidation of KI by
iodate may be designated as shown below :
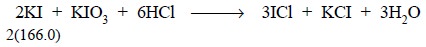
The reduction of KIO3 may be expressed as :
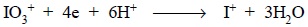
Hence, from the above equation we have, 1 mol of KIO3
is 4 equivalent and a 0.05 M solution would be 0.2 N.
Thus, we have :
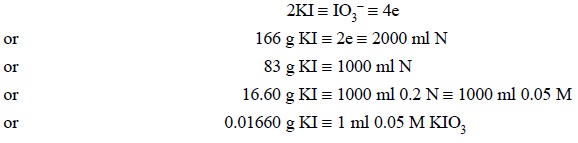
Materials Required : Potassium iodide : 0.5 g ;
hydrochloric acid ( −~ 11.5 N) : 35 ml ; chloroform : 5 ml ;
0.05 M potassium iodate.
Procedure : Weigh accurately 0.5 g of
potassium iodide and dissolve it in about 10 ml of DW. Add to it 35 ml of hydrochloric acid and 5 ml
of chloroform. Titrate with 0.05 M potassium iodate till the purple colour of
iodine disappears from the chloroform layer. Add the last portion of the iodate
solution carefully and dropwise while shaking the contents of the flask
vigorously and continuously. Allow to stand for 5 minutes. In case any colour
still develops in the chloroform layer continue the titration. Each ml of 0.05
M potassium iodate is equivalent to 0.0166 g of potassium iodide.
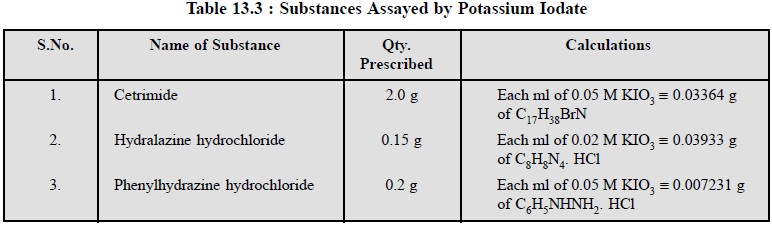
3.4. Cognate Assays
A host of other pharmaceutical substances, namely :
cetrimide, hydralazine hydrochloride, phenylhydrazine hydrochloride may be
assayed by titration with potassium iodate as mentioned in Table : 13.3.
Related Topics