Chapter: Pharmaceutical Drug Analysis: Karl Fischer Method for Determination of Water
Applications of Karl Fischer Method for Determination of Water in Pharmaceutical Analysis
APPLICATIONS OF KARL FISCHER METHOD FOR DETERMINATION OF WATER IN
PHARMACEUTICAL ANALYSIS
The Karl Fischer method for the determination of water is
used for prednisolone sodium phosphate as described below.
1. PREDNISOLONE SODIUM PHOSPHATE
Materials Required : Karl Fischer Reagent* : 100 ml
; prednisolone sodium phosphate : 0.2 g ; anhydrous methanol : 20.0 ml.
Procedure : Add about 20 ml of anhydrous
methanol to the titration vessel and titrate to the amperometric end-point with the Karl Fischer
reagent. Quickly add 0.2 g of prednisolone sodium phosphate sample, stir for 1
minute and again titrate to the amperometric end-point with the Karl Fischer
reagent. The difference between the two titrations gives the volume (v) of Karl Fischer reagent consumed by
the sample.
The minimum water equivalent is 3.5 mg of water per ml of
Karl Fischer reagent. Hence, the percentage of water w/w in the given sample
may be calculated by the following expression :
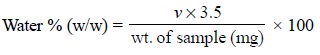
Precautions :
·
The reagents and solutions used must be kept anhydrous
and necessary care should be taken through-out to prevent exposure to
atmospheric moisture,
·
The Karl Fischer reagent should be protected from light
and preferably stored in a bottle fitted with an automatic burette, and
·
The water equivalent of Karl Fischer reagent should
always be determined before use.
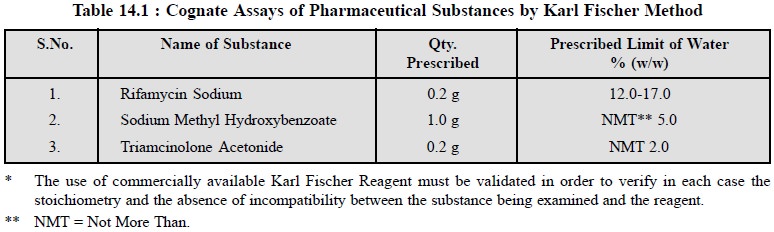
2. COGNATE ASSAYS
A number of other official
pharmaceutical substances may be assayed for their water content by the
Karl Fischer method as summarized in the following Table 14.1.
Related Topics