Chapter: Pharmaceutical Drug Analysis: Karl Fischer Method for Determination of Water
Instrumentation - Karl Fischer Method for Determination of Water
INSTRUMENTATION
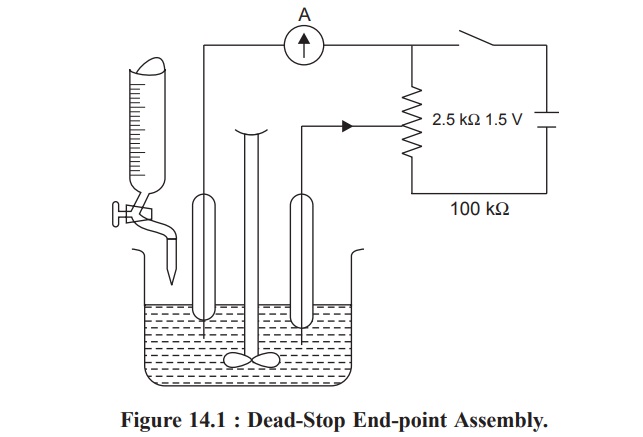
Figure 14.1 illustrates a simple dead-stop end-point
assembly or a Karl Fischer titration apparatus. The titration vessel is fitted
with a pair of identical platinum electrodes, a mechanical stirrer with
adjustable speed, and a burette. It will be observed that absolutely little or
no current may flow unless and until the solution is totally free from any
polarizing substances ; this could perhaps be due to the absorbed layers of
oxygen and hydrogen on the anode and cathode respectively. However, the current
shall flow only when the two electrodes get depolarized. The Karl Fischer
reagent is pumped into the burette by means of hand bellows, the eccess of
moisture is usually prevented by employing an appropriate arrangement of
desiccant tubes. Alternatively, the stirring may also be accomplished either by
using a magnetic stirrer or by means of a suitably dried nitrogen passed gently
through the solution during the course of titration.
The end-point is achieved by employing an eiectrical
circuit comprising of a microammeter (A), platinum electrodes, together with a
1.5 V to 2.0 V battery connected across a variable resistance of about 2.5 kΩ. First of all the resistance
is adjusted in such a manner that an initial current passes through the
platinum electrodes in series with a microammeter (A). After each addition of
reagent, the pointer of the microammeter gets deflected but quickly returns to
its original position. At the end of the reaction a deflection is obtained
which persists for 10-15 seconds.
1. AUTOMATED ELECTROCHEMICAL KARL FISCHER ANALYSIS
Commercially available Modern KF-Titrators are usually
equipped with specifically designed titration vessels that are exclusively
meant to check and prevent the contact with atmospheric moisture. Quite a few
such devices are armed with microprocessors that will perform the requisite
operations sequentially in a programmed manner automatically ; and may also
dish out a print-out of the desired results including the percentage moisture
content. In fact, these Modern KF-Titrators not only afford greater accuracy
and precision in results but also offer much ease and convenience in routine
analysis as compared to the classical techniques based on either caulometry or
controlled current potentiometry using two indicator electrodes.
In this procedure the iodide needed for the reaction with
water is normally generated within the titration vessel caulometrically as shown below :
H2O + I2 + SO2 + 3C5H5N + CH3OH → 2C5H5N.HI + C5H5NH.SO4.CH3
Thus, the basis of the analysis rests upon the
quantitative relationship existing between charge passed and iodine produced by
the reagent according to the above reaction. Therefore, the generation of
iodine is automatically stopped when an excess of it is detected by the
indicator electrode. It essentially consists of two platinum electrodes across
which an AC is applied and subsequently a marked drop in voltage between the
electrodes takes place as soon as an excess of iodine is present. Normally such
automated instruments make use of proprietory
reagents exclusively.
The major advantage of this approach to KF-analysis being
that no calibration is required as the method is absolute and is entirely based
on the stoichiometry of the aforesaid equation. It is noteworthy that one may
determine the amounts of water ranging between 10 mcg and 10 mg in solid as
well as liquid samples.
Related Topics