Chapter: 9th Science : Atomic Structure
Isotopes (Iso – same, topo – place, Isotope – same place
Isotopes (Iso – same, topo – place, Isotope – same place
1. ISOTOPES
Find below three different atoms. Count the
different subatomic particles and fill in the table.
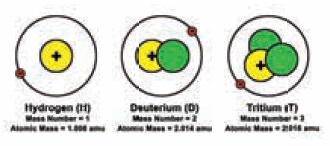
What do you observe in the above atoms?
Which is same and what is different in them?
All of them have the same number of protons and
electrons but different number of neutrons.
What will they have in common?
All the three structures have same atomic number
but different mass numbers. They have the same number of electrons also. Such
atoms of the same element are called isotopes.
Isotopes
are atoms of the same element having same atomic number but different mass
numbers.
This is due to the difference in the number of
neutrons in the nucleus. Isotopes differ in few physical properties such as
density, boiling point etc. Physical properties depend upon mass number.
Isotopes have different mass numbers. So they differ in physical properties.
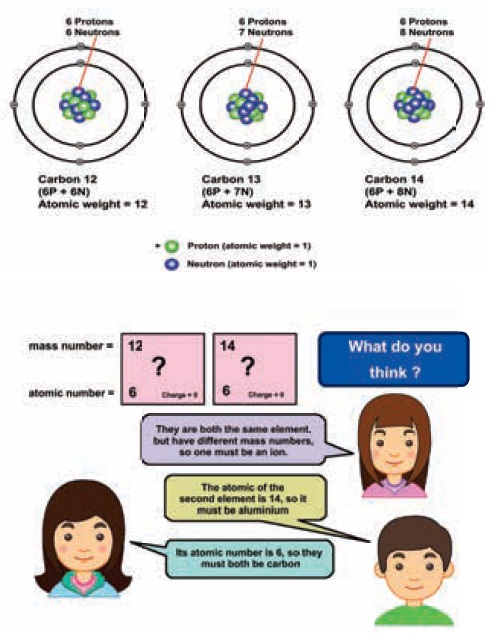
Example: Isotopes of carbon
Why do some isotopes show radioactivity?
When the number of neutrons exceeds the number of
protons in the nucleus of atoms, some nuclei become unstable. These unstable
nuclei break up spontaneously emitting certain type of radiations. They are
known as radioactive isotopes. Examples: H3and C14
Many elements have isotopes of which some of them
are radioactive isotopes.
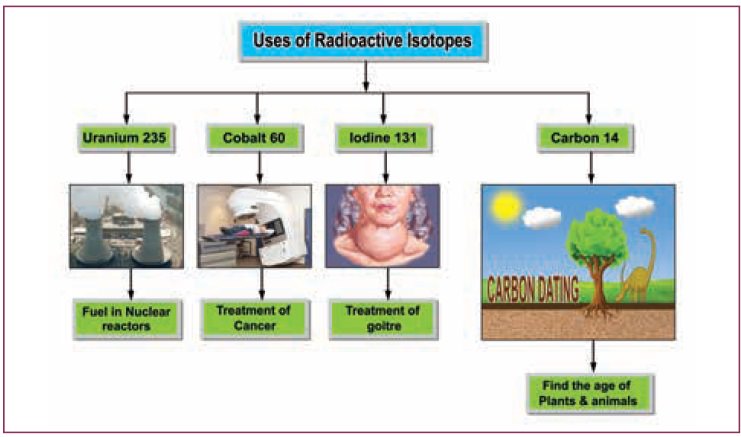
Uses of radioactive isotopes
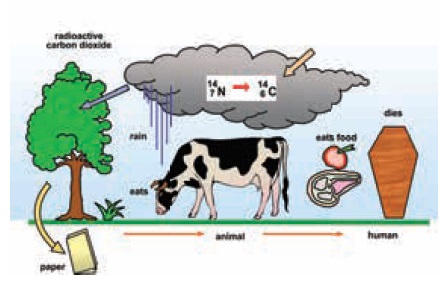
Radioactivity prevails around us. The food we eat,
the air we breathe, the buildings we live in, all contain small amounts of
radioactive materials. This radiation will be present always.
Thus there are a lot of low level natural
radioactivity around us. For example, our bodies contain radioisotopes, such as
potassium-40, which continuously emit radiation, but the level is so low that
this does not harm us.The picture below shows us how radioactive carbon(C14) is
all around us.
But the special properties of radioactive isotopes
make them useful to us in various fields.
2. Isobars

What is the difference between these two atoms?
The above two elements calcium and argon have
atomic number 20 and atomic number 18 respectively. is means they have different
number of protons and electrons. But the mass number of both these elements is
40. It follows that the total number of nucleons in both of them are the same.
Atoms of different elements with different atomic numbers, which have the same
mass number, are known as isobars.
3. Isotones
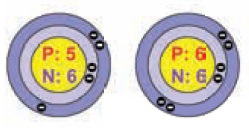
The above pair of elements Boron and Carbon has the
same number of neutrons but different number of protons and hence different
atomic numbers. Atoms of different elements with different atomic numbers and
different mass numbers, but with the same number of neutrons are called
isotones.
How are electrons arranged around the nucleus in an atom?
So far we have been discussing about the nucleus of
an atom and the protons and neutrons which constitute the nucleus. We also saw
that electrons are extra nuclear particles and they revolve around the nucleus
in fixed trajectories or orbits. Let us now see how electrons are arranged in
different orbits. The systematic arrangement of electrons in various shells or
orbits in an atom is called the electronic configuration.
Electronic configuration of atoms:
You already know that electrons occupy different
energy levels called orbits or shells.The distribution of electrons in these
orbits of an atom is governed by certain rules or conditions. These are known
as Bohr and Bury Rules of electronic configuration.
Bohr and Bury simultaneously proposed the following
rules for the distribution of electrons in different shells.
·
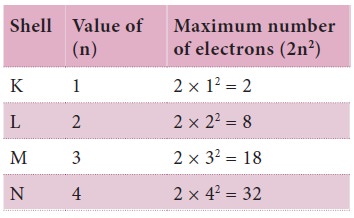
Rule 1: The maximum number of electrons that can be accommodated in a shell is equal to 2n2
where ‘n’ is the quantum number of the shell (i.e., the serial number of the
shell from the nucleus).
·
Rule 2: Shells are filled in a stepwise manner in the increasing order of energy.
·
Rule 3: The outermost shell cannot have more than 8 electrons and the next inner, i.e., the
penultimate shell cannot have more than 18 electrons.
Illustration:
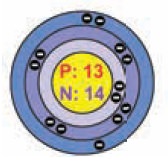
Structure of Aluminium atom: (13 electrons) K shell = 2 electron, L shell = 8, M Shell
– 3
So its
electronic con guration is 2, 8, 3
Electronic configuration of first 20 elements
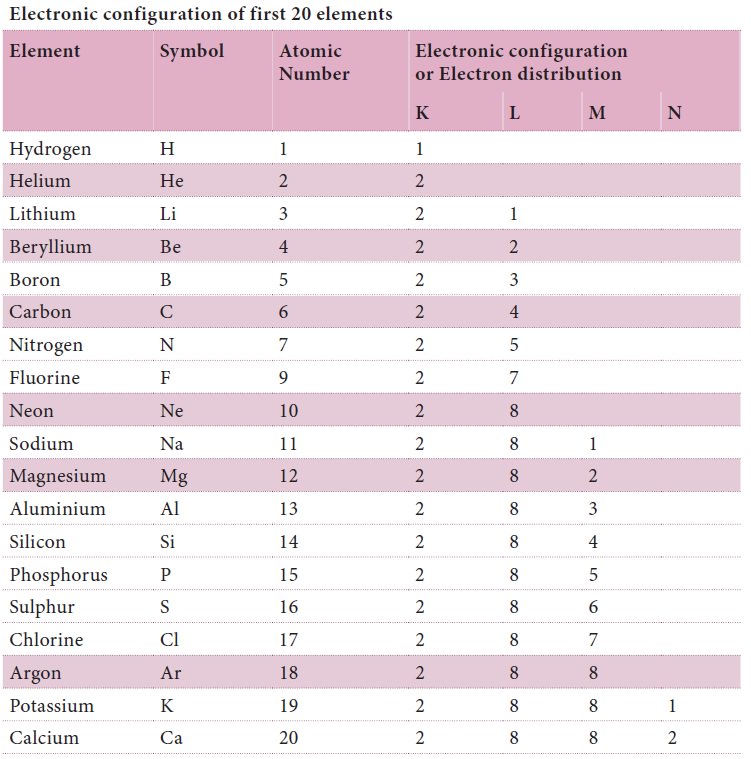
For getting a basic idea about the electron
distribution around the nucleus we can draw schematic diagrams as shown below.
As you learn more about atomic structure you will come to know that the real
picture of electron distribution is entirely different from what we have shown
here.
Schematic diagrams for Atomic Structure of Elements
(first 20)
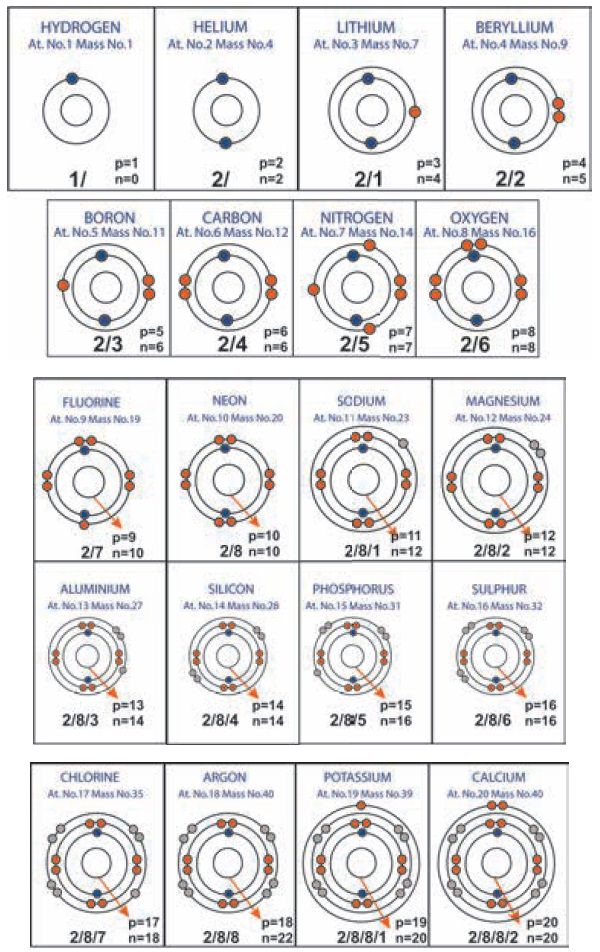
4. Valence electrons
How many electrons are in the outermost shell? 5
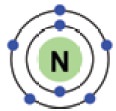
The outermost shell of an atom is called its
valence shell and the electrons present in the valence shell are known as
valence electrons.
Hydrogen atom has only one electron in its valence
shell. Hence it has one valence
electron. Similarly carbon has 4 electrons in the outermost shell and so it has
4 valence electrons.
The chemical properties of elements are decided by
these valence electrons, since they are the ones that take part in chemical
reaction.
The elements with same number of electrons in the
valence shell show similar properties and those with different number of
valence electrons show different chemical properties. Elements, which have
valence electrons 1 or 2 or 3 (except Hydrogen) are metals.
These elements can lose electrons to form ions
which are positively charged and are called cations.
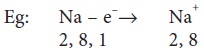
Elements with 4 to 7 electrons in their valence
shells are non-metals.
These elements can gain electrons to form ions
which are negatively charged and are called anions.
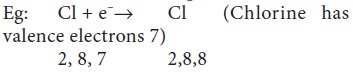
5. Valency
Valency of an element is the combining capacity of
the element with other elements and is equal to the number of electrons that
take part in a chemical reaction. Valency of the elements having valence
electrons 1, 2, 3, 4 is 1, 2, 3, 4 respectively.
While valency of an element with 5, 6 &7
valence electrons is 3, 2 and 1 (8–valence electrons) respectively, where 8 is
the number of electrons required by an element to attain stable electronic con
guration. Elements having completely lled outermost shell show Zero valency.
For example: e electronic con guration of Neon is
2,8 (completely lled). So valency is 0.
Illustration:
Assign the valency of Magnesium & Sulphur
Electronic configuration of magnesium is 2, 8, 2.
So valency is 2.
Electronic configuration of sulphur is 2, 8, 6. So valency is 2 i.e.(8 – 6)
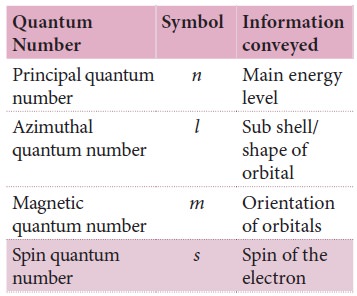
Introduction to Quantum Numbers
We have learnt to designate orbits (shells) by
K,L,M, N …., and orbitals (sub shells) as s, p, d and f. We have seen that
electrons are lled in to these orbitals according to certain rules. Can we now
designate an electron in an atom in a manner in which it gets a unique
identity? Each electron inside of an atom has its own ‘identity’ which is given
by four quantum numbers that
communicate a great deal of
information about that electron.
The
numbers which designate and distinguish various atomic orbitals and electrons
present in an atom are called quantum numbers.
How would you describe to someone exactly where you
live? I guess you would start with your address. (similar to the identity fo an
electron).
When you
specify the location of a building, you usually list which country it is in,
which state and city it is in that country, then the area and the street and
the door number.
Just like no two buildings have the exact same
address, no two electrons can have the same set of quantum numbers.
A quantum
number describes a speci c aspect of an electron. Just like we have four
ways of de ning the location of a building (country, state, city, and street
address), we have four ways of de ning the properties of an electron, i.e.four quantum numbers.
These quantum numbers tell us
·
how far is the electron from the
nucleus,-(Principal Quantum number)
·
which orbital does it occupy and what
is its shape (Azimuthal
Quantum number)
·
how this orbital is oriented in space (Magnetic Quantum number)
·
what kind of spin the electron has. (Spin Quantum number):
You will learn more details about this in higher
classes.
Related Topics