Chapter: Basic & Clinical Pharmacology : Antimycobacterial Drugs
Isoniazid - Drugs Used In Tuberculosis
ISONIAZID
Isoniazid is the most
active drug for the treatment of tuberculosis caused by susceptible strains. It
is a small molecule (MW 137) that is freely soluble in water. The structural
similarity to pyridoxine is shown below.
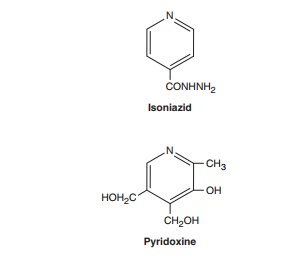
In vitro, isoniazid inhibits most tubercle bacilli at a concentra-tion of 0.2 mcg/mL or less and is bactericidal for actively growing tubercle bacilli. It is less effective against atypical mycobacterial species. Isoniazid penetrates into macrophages and is active against both extracellular and intracellular organisms.
Mechanism of Action & Basis of Resistance
Isoniazid inhibits
synthesis of mycolic acids, which are essential components of mycobacterial
cell walls. Isoniazid is a prodrug that is activated by KatG, the mycobacterial
catalase-peroxidase. The activated form of isoniazid forms a covalent complex
with an acyl carrier protein (AcpM) and KasA, a beta-ketoacyl carrier protein
synthetase, which blocks mycolic acid synthesis and kills the cell. Resistance
to isoniazid is associated with mutations resulting in overexpression of inhA, which encodes an NADH-dependent
acyl carrier protein reductase; mutation or deletion of the katG gene; promoter mutations resulting
in overexpression of ahpC, a
puta-tive virulence gene involved in protection of the cell from oxida-tive
stress; and mutations in kasA.
Overproducers of inhA express
low-level isoniazid resistance and cross-resistance to ethionamide. KatG mutants express high-level
isoniazid resistance and often arenot cross-resistant to ethionamide.
Drug-resistant mutants
are normally present in susceptible mycobacterial populations at about 1
bacillus in 106. Since tubercu-lous
lesions often contain more than 108 tubercle bacilli, resistant mutants are
readily selected if isoniazid or any other drug is given as a single agent. The
use of two independently acting drugs in com-bination is much more effective.
The probability that a bacillus is initially resistant to both drugs is
approximately 1 in 106× 106, or 1 in 1012, several orders of magnitude greater than the
number of infecting organisms. Thus, at least two (or more in certain cases)
active agents should always be used to treat active tuberculosis to prevent
emergence of resistance during therapy.
Pharmacokinetics
Isoniazid
is readily absorbed from the gastrointestinal tract. A 300-mg oral dose (5
mg/kg in children) achieves peak plasma concentrations of 3–5 mcg/mL within 1–2
hours. Isoniazid dif-fuses readily into all body fluids and tissues. The
concentration in the central nervous system and cerebrospinal fluid ranges
between 20% and 100% of simultaneous serum concentrations.Metabolism of
isoniazid, especially acetylation by liver N-acetyltransferase,
is genetically determined .The average plasma concentration of isoniazid in
rapid acetylators is about one third to one half of that in slow acetylators,
and aver-age half-lives are less than 1 hour and 3 hours, respectively. More
rapid clearance of isoniazid by rapid acetylators is usually of no therapeutic
consequence when appropriate doses are administered daily, but subtherapeutic
concentrations may occur if drug is administered as a once-weekly dose or if
there is malabsorption.
Isoniazid
metabolites and a small amount of unchanged drug are excreted, mainly in the
urine. The dose need not be adjusted in renal failure. Dose adjustment is not
well defined in patients with severe preexisting hepatic insufficiency
(isoniazid is contrain-dicated if it is the cause of the hepatitis) and should
be guided by serum concentrations if a reduction in dose is contemplated.
Clinical Uses
The typical dosage of
isoniazid is 5 mg/kg/d; a typical adult dose is 300 mg given once daily. Up to
10 mg/kg/d may be used for serious infections or if malabsorption is a problem.
A 15 mg/kg dose, or 900 mg, may be used in a twice-weekly dosing regimen in
combination with a second antituberculous agent (eg, rifampin 600 mg).
Pyridoxine, 25–50 mg/d, is recommended for those with conditions predisposing
to neuropathy, an adverse effect of isoniazid. Isoniazid is usually given by
mouth but can be given parenterally in the same dosage.
Isoniazid as a single
agent is also indicated for treatment of latent tuberculosis. The dosage is 300
mg/d (5 mg/kg/d) or 900 mg twice weekly for 9 months.
Adverse Reactions
The
incidence and severity of untoward reactions to isoniazid are related to dosage
and duration of administration.
A. Immunologic Reactions
Fever and skin rashes
are occasionally seen. Drug-induced sys-temic lupus erythematosus has been
reported.
B. Direct Toxicity
Isoniazid-induced
hepatitis is the most common major toxic effect. This is distinct from the
minor increases in liver aminotransferases (up to three or four times normal),
which do not require cessation of the drug and which are seen in 10–20% of
patients, who usu-ally are asymptomatic. Clinical hepatitis with loss of
appetite, nausea, vomiting, jaundice, and right upper quadrant pain occurs in
1% of isoniazid recipients and can be fatal, particularly if the drug is not
discontinued promptly. There is histologic evidence of hepatocellular damage
and necrosis. The risk of hepatitis depends on age. It occurs rarely under age
20, in 0.3% of those aged 21–35, 1.2% of those aged 36–50, and 2.3% for those
aged 50 and above. The risk of hepatitis is greater in individuals with
alco-hol dependence and possibly during pregnancy and the postpar-tum period.
Development of isoniazid hepatitis contraindicates further use of the
drug.Peripheral neuropathy is observed in 10–20% of patients given dosages
greater than 5 mg/kg/d, but it is infrequently seen with the standard 300-mg adult
dose. Peripheral neuropathy is more likely to occur in slow acetylators and
patients with predisposing conditions such as malnutrition, alcoholism,
diabetes, AIDS, and uremia. Neuropathy is due to a relative pyridoxine
deficiency. Isoniazid promotes excretion of pyridoxine, and this toxicity is
readily reversed by administration of pyridoxine in a dosage as low as 10 mg/d.
Central nervous system toxicity, which is less com-mon, includes memory loss,
psychosis, and seizures. These effects may also respond to pyridoxine.
Miscellaneous other
reactions include hematologic abnormali-ties, provocation of pyridoxine
deficiency anemia, tinnitus, and gastrointestinal discomfort. Isoniazid can
reduce the metabolism of phenytoin, increasing its blood level and toxicity.
Related Topics