Chapter: Medical Surgical Nursing: Management of Patients With Cerebrovascular Disorders
Ischemic Stroke
Ischemic Stroke
Approximately 400,000 people have an ischemic
stroke in the United States each year (Hock, 1999). An ischemic stroke, cere-brovascular
accident (CVA), or what is now being termed “brain attack” is a sudden loss of
function resulting from disruption of the blood supply to a part of the brain.
This event is usually the result of long-standing cerebrovascular disease. The
term “brain attack” is being used to suggest to health care practitioners and
the public that a stroke is an urgent health care issue similar to a heart
attack. This change in terms also reflects a similar manage-ment strategy in
both diseases. Early treatment results in fewer symptoms and less loss of
function. Only 8% of ischemic strokes result in death within 30 days (American
Heart Association, 2000).
The net lifetime stroke-related costs in patients
over the age of 65 with a first ischemic stroke are estimated at $62,000
($45,000 direct costs plus $17,000 indirect costs). The cost for younger
patients (those less than 65 years) is even greater, at $198,000 per year
($65,000 direct costs plus $133,000 indirect costs). The approximate annual
cost in the United States for ischemic stroke care is over $71.8 billion
(Matchar & Samsa, 2000).
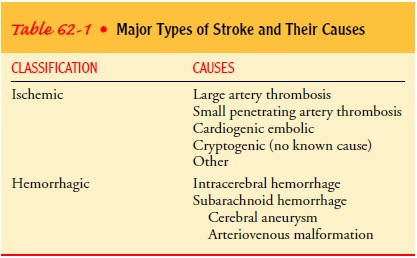
Ischemic
strokes are subdivided into five different types ac-cording to their cause:
large artery thrombosis (20%), small pen-etrating artery thrombosis (25%),
cardiogenic embolic stroke (20%), cryptogenic (30%) and other (5%) (see Table
62-1).
Large
artery thrombotic strokes are due to atherosclerotic plaques in the large blood
vessels of the brain. Thrombus forma-tion and occlusion at the site of the
atherosclerosis result in is-chemia and infarction.
Small
penetrating artery thrombotic strokes affect one or more vessels and are the
most common type of ischemic stroke. Small artery thrombotic strokes are also
called lacunar strokes be-cause of the cavity that is created once the
infarcted brain tissue disintegrates.
Cardiogenic
embolic strokes are associated with cardiac dys-rhythmias, usually atrial
fibrillation. Emboli originate from the heart and circulate to the cerebral
vasculature, most commonly the left middle cerebral artery, resulting in a
stroke. Embolic strokes may be prevented by the use of anticoagulation therapy
in patients with atrial fibrillation.
The
last two classifications of ischemic strokes are cryptogenic strokes, which have
no known cause, and other strokes, from causes such as cocaine use,
coagulopathies, migraine, and spon-taneous dissection of the carotid or
vertebral arteries (Hock, 1999; Schievink, 2001).
Pathophysiology
In an ischemic brain attack, there is disruption of the cerebral blood flow due to obstruction of a blood vessel. This disruption in blood flow initiates a complex series of cellular metabolic events referred to as the ischemic cascade (Fig. 62-1).
The
ischemic cascade begins when cerebral blood flow falls to less than 25 mL/100
g/min. At this point, neurons can no longer maintain aerobic respiration. The
mitochondria must then switch to anaerobic respiration, which generates large
amounts of lactic acid, causing a change in the pH level. This switch to the
less efficient anaerobic respiration also renders the neuron inca-pable of
producing sufficient quantities of adenosine triphosphate (ATP) to fuel the
depolarization processes. Thus, the membrane pumps that maintain electrolyte
balances begin to fail and the cells cease to function.
Early in the cascade, an area of low cerebral blood
flow, re-ferred to as the penumbra region, exists around the area of
in-farction. The penumbra region is
ischemic brain tissue that can be salvaged with timely intervention. The
ischemic cascade threatens cells in the penumbra because membrane
depolariza-tion of the cell wall leads to an increase in intracellular calcium
and the release of glutamate (Hock, 1999). The penumbra area can be revitalized
by administration of tissue plasminogen acti-vator (t-PA), and the influx of
calcium can be limited with the use of calcium channel blockers. The influx of
calcium and the re-lease of glutamate, if continued, activate a number of
damaging pathways that result in the destruction of the cell membrane, the
release of more calcium and glutamate, vasoconstriction, and the generation of
free radicals. These processes enlarge the area of in-farction into the
penumbra, extending the stroke.
Each
step in the ischemic cascade represents an opportunity for intervention to
limit the extent of secondary brain damage caused by a stroke. Medications that
protect the brain from sec-ondary injury are called neuroprotectants (Reed,
2000). A num-ber of clinical trials are focusing on calcium channel antagonists
that block the calcium influx, glutamate antagonists, antioxidants, and other
neuroprotectant strategies that will help prevent sec-ondary complications
(NINDS, 1999; Reed, 2000).
Clinical Manifestations
An ischemic stroke can cause a wide variety of
neurologic deficits, depending on the location of the lesion (which vessels are
ob-structed), the size of the area of inadequate perfusion, and the amount of
collateral (secondary or accessory) blood flow. The pa-tient may present with
any of the following signs or symptoms:
·
Numbness or weakness of the
face, arm, or leg, especially on one side of the body
·
Confusion or change in mental
status
·
Trouble speaking or
understanding speech
·
Visual disturbances
·
Difficulty walking, dizziness,
or loss of balance or coordi-nation
·
Sudden severe headache
Motor,
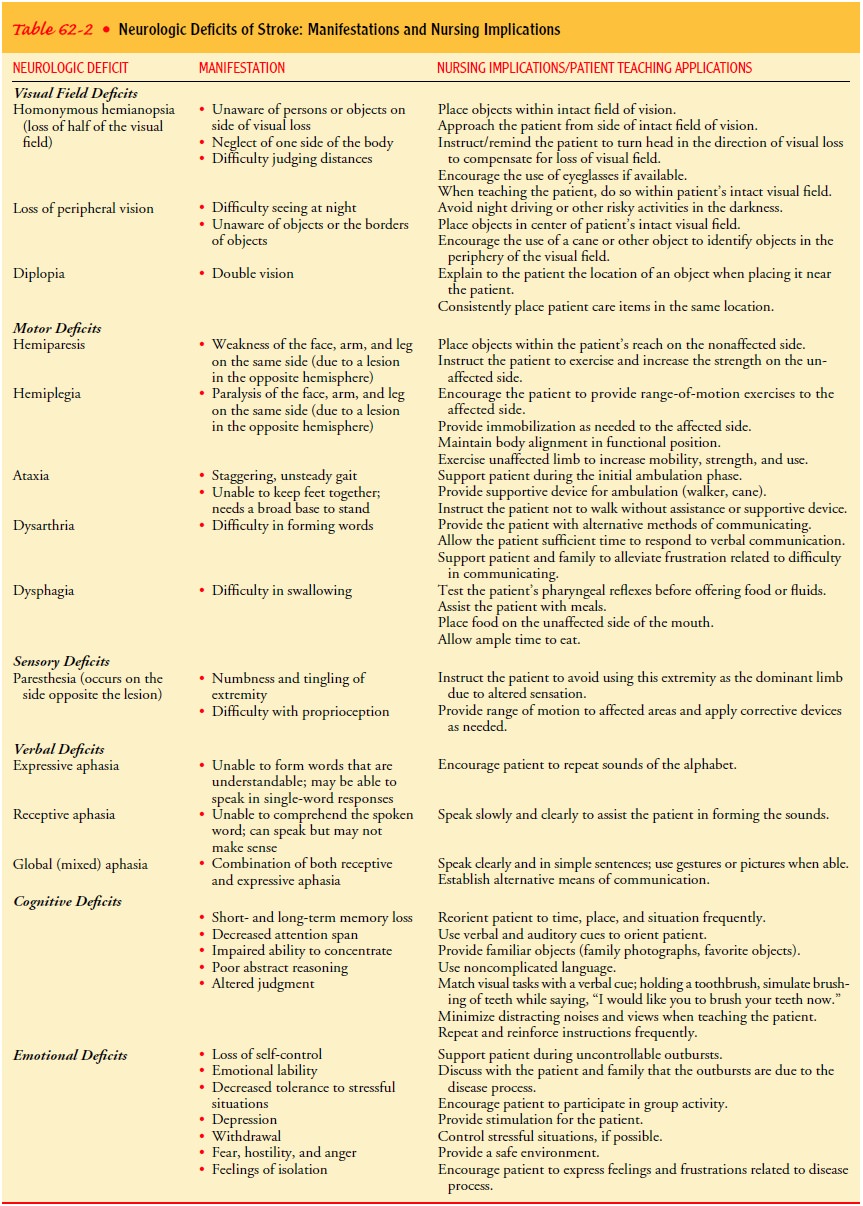
sensory, cranial nerve, cognitive, and other functions may be disrupted. Table
62-2 reviews the neurologic deficits fre-quently seen in patients with strokes.
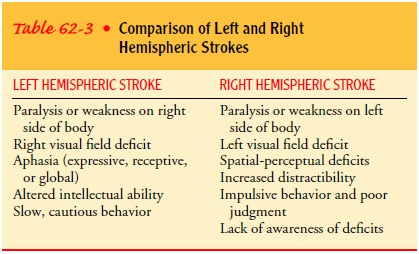
Table 62-3 compares the symptoms seen in right hemispheric stroke with those
seen in left hemispheric stroke. Patients exhibit deficits in specific
locations as well as different behavior.
MOTOR LOSS
A stroke is a lesion of the upper motor neurons and
results in loss of voluntary control over motor movements. Because the upper
motor neurons decussate (cross), a disturbance of voluntary motor control on
one side of the body may reflect damage to the upper motor neurons on the
opposite side of the brain. The most com-mon motor dysfunction is hemiplegia (paralysis of one side of
the body) due to a lesion of the opposite side of the brain. Hemi-paresis, or weakness of one side
of the body, is another sign.
In the early stage of stroke, the initial clinical
features may be flaccid paralysis and loss of or decrease in the deep tendon
re-flexes. When these deep reflexes reappear (usually by 48 hours), increased
tone is observed along with spasticity (abnormal in-crease in muscle tone) of
the extremities on the affected side.
COMMUNICATION LOSS
Other brain functions affected by stroke are
language and com-munication. In fact, stroke is the most common cause of
aphasia. The following are dysfunctions of language and communication:
·
Dysarthria
(difficulty in speaking), caused by paralysis ofthe
muscles responsible for producing speech
·
Dysphasia or aphasia (defective speech or loss of
speech), which can be expressive aphasia,
receptive aphasia, or global (mixed)
aphasia
·
Apraxia
(inability to perform a previously learned action),as may
be seen when a patient picks up a fork and attempts to comb his hair with it
PERCEPTUAL DISTURBANCES
Perception is the ability to interpret sensation.
Stroke can result in visual-perceptual dysfunctions, disturbances in
visual-spatial relations, and sensory loss.
Visual-perceptual dysfunctions are due to
disturbances of the primary sensory pathways between the eye and visual cortex.
Homonymous hemianopsia (loss of half
of the visual field) may occur from stroke and may be temporary or permanent.
The af-fected side of vision corresponds to the paralyzed side of the body.
Disturbances in visual-spatial relations (perceiving the relation of two or more objects in spatial areas) are frequently seen in pa-tients with right hemispheric damage.
SENSORY LOSS
The sensory losses from stroke may take the form of
slight im-pairment of touch or may be more severe, with loss of proprio-ception
(ability to perceive the position and motion of body parts) as well as
difficulty in interpreting visual, tactile, and audi-tory stimuli.
COGNITIVE IMPAIRMENT AND PSYCHOLOGICAL EFFECTS
If damage has occurred to the frontal lobe,
learning capacity, memory, or other higher cortical intellectual functions may
be impaired. Such dysfunction may be reflected in a limited atten-tion span,
difficulties in comprehension, forgetfulness, and a lack of motivation, which
cause these patients to become frustrated in their rehabilitation program.
Depression is common and may be exaggerated by the patient’s natural response
to this catastrophic event. Other psychological problems are common and are
man-ifested by emotional lability, hostility, frustration, resentment, and lack
of cooperation.
Assessment and Diagnostic Findings
Any patient with neurologic deficits needs a
careful history and a complete physical and neurologic examination. Initial
assessment will focus on airway patency, which may be compromised by loss of
gag or cough reflexes and altered respiratory pattern; cardio-vascular status
(including blood pressure, cardiac rhythm and rate, carotid bruit), and gross
neurologic losses.
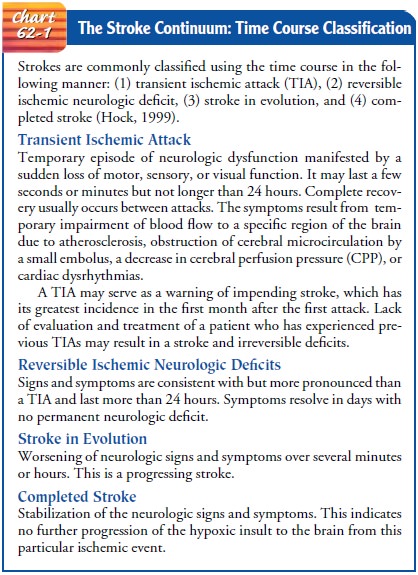
Stroke patients may present to the acute care
facility at any point along a continuum of neurologic involvement. A system
that uses the time course to classify patients along this continuum may be used
to guide treatment. Strokes using the time course are com-monly classified in
the following manner: (1) transient ischemic attack (TIA); (2) reversible ischemic
neurologic deficit; (3) stroke in evolution; and (4) completed stroke (Hock,
1999) (Chart 62-1).
The initial diagnostic test for a stroke is a
noncontrast com-puted tomography (CT) scan performed emergently to deter-mine
if the event is ischemic or hemorrhagic (which determines treatment). Further
diagnostic workup for ischemic stroke in-volves attempting to identify the
source of the thrombi or emboli. A 12-lead electrocardiogram and a carotid
ultrasound are stan-dard tests. Other studies may include cerebral angiography,
trans-cranial Doppler flow studies, transthoracic or transesophageal
echocardiography, magnetic resonance imaging of the brain and/or neck, xenon
CT, and single photon emission CT (Bonnono et al., 2000; Petty et al., 2000).
In a patient with a TIA, a bruit (abnormal sound heard on aus-cultation resulting from interference with normal blood flow) may be heard over the carotid artery. There are diminished or absent carotid pulsations in the neck.
Diagnostic
tests for TIA may include carotid phonoangiography; this involves auscultation,
di-rect visualization, and photographic recording of carotid bruits.
Oculoplethysmography measures the pulsation of blood flow through the
ophthalmic artery. Carotid angiography allows visu-alization of intracranial
and cervical vessels. Digital subtraction angiography is used to define carotid
artery obstruction and pro-vides information on patterns of cerebral blood
flow.
Prevention
Primary prevention of ischemic stroke is the best
approach. Stroke risk screenings are an ideal opportunity to lower stroke risk
by identifying high-risk individuals or groups and educating the pa-tients and
the community about recognition and prevention of stroke (Lindsey, 2000;
Manzella & Galante, 2000).
Advanced age, gender, and race are well-known
non-modifiable risk factors for stroke (American Heart Association, 2000).
Specifically, high-risk groups include people over the age of 55, because the
incidence of stroke more than doubles in each suc-cessive decade, and men, who
have a higher rate of stroke than women (due to the higher prevalence of women
in the elderly population, however, the absolute numbers of men and women with
stroke are similar). Another high-risk group is African Amer-icans: the
incidence of first stroke in African Americans is almost twice that in
Caucasians. African Americans also suffer more extensive physical impairments
and are twice as likely to die from stroke than Caucasians. Hispanic, Native
American Indian, Alaska native, and Asian/Pacific Islander ethnic groups also
have a higher relative risk of stroke compared to Caucasians.
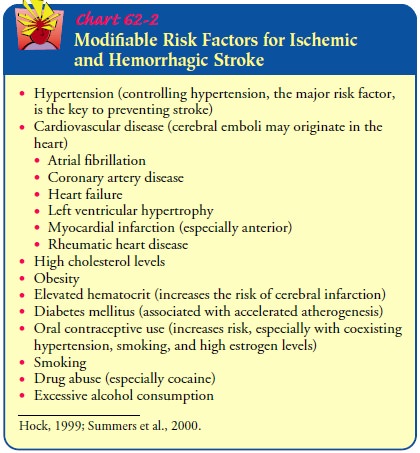
Modifiable risk factors for ischemic stroke include
hyperten-sion, cardiovascular disease, high cholesterol, obesity, smoking, and
diabetes (Chart 62-2). For people at high risk, interventions that alter
modifiable factors, such as treating hypertension and hyperglycemia and
stopping smoking, will reduce stroke risk. Many health promotion efforts
involve encouraging a healthy lifestyle, including eating a low-fat,
low-cholesterol diet and in-creasing exercise. Recent evidence suggests that
eating fish two or more times per week reduces the risk of thrombotic stroke
for women (Iso et al., 2001).
Several methods of preventing recurrent stroke have
been identified for patients with TIAs or mild ischemic stroke. Patients with
moderate to severe carotid stenosis are treated with carotid endarterectomy
(Wolf et al., 1999). In patients with atrial fibril-lation, which increases the
risk of emboli, administration of war-farin (Coumadin), an anticoagulant that
inhibits clot formation, may prevent both thrombotic and embolic strokes.
Medical Management
Patients who have experienced a TIA or mild stroke from atrial fibrillation or from suspected embolic or thrombotic causes are candidates for nonsurgical medical management. Those with atrial fibrillation are treated with dose-adjusted warfarin sodium (Coumadin) unless contraindicated. The INR target is 2.5. When warfarin is contraindicated, aspirin is used in doses be-tween 50 and 325 mg/d (Wolf et al., 1999).
Platelet-inhibiting medications (aspirin,
dipyridamole [Per-santine], clopidogrel [Plavix], and ticlopidine [Ticlid])
decrease the incidence of cerebral infarction in patients who have experi-enced
TIAs from suspected embolic or thrombotic causes. Cur-rently the most
cost-effective antiplatelet regimen is aspirin 50 mg/d and dipyridamole 400
mg/d (Sarasin et al., 2000).
THROMBOLYTIC THERAPY
Thrombolytic agents are used to treat ischemic
stroke by dis-solving the blood clot that is blocking blood flow to the brain.
Recombinant t-PA is a genetically engineered form of t-PA, a thrombolytic
substance made naturally by the body. It works by binding to fibrin and
converting plasminogen to plasmin, which stimulates fibrinolysis of the
atherosclerotic lesion. Rapid diag-nosis of stroke and initiation of
thrombolytic therapy (within 3 hours) in patients with ischemic stroke leads to
a decrease in the size of the stroke and an overall improvement in functional
out-come after 3 months (NINDS t-PA Stroke Study Group, 1995). To realize the
full potential of thrombolytic therapy, community education directed at
recognizing the symptoms of stroke and obtaining appropriate emergency care is
necessary to ensure rapid transport to a hospital and initiation of therapy
within the 3-hour time frame (Manzella & Galante, 2000). Delays make the
patient ineligible for thrombolytic therapy because revascularization of
necrotic tissue (which develops after 3 hours) increases the risk for cerebral
edema and hemorrhage.
Enhancing Prompt Diagnosis.
After being notified by emergencymedical service
personnel, the emergency department calls the appropriate staff (neurologist,
neuroradiologist, radiology de-partment, nursing staff, and electrocardiogram
technician) and informs them of the patient’s imminent arrival at the hospital.
Many institutions have brain attack teams that respond rapidly, ensuring that
treatment occurs within the allotted time frame (Alberts et al., 2000; Bonnono
et al., 2000).
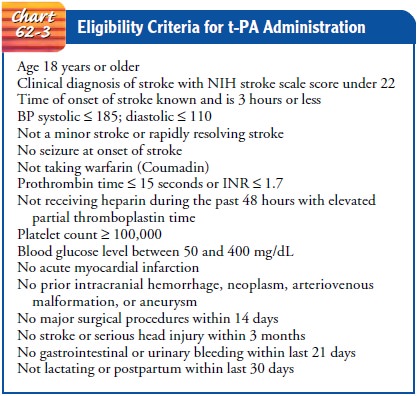
Initial management requires the definitive
diagnosis of an is-chemic stroke by CT scanning and determination of whether
the patient meets all the criteria for t-PA therapy (Chart 62-3). Some of the
contraindications for thrombolytic therapy include symp-tom onset greater than
3 hours prior to admission, a patient who is anticoagulated, a patient who has
had a recent myocardial in-farction, or a patient who has had any type of
intracranial pathol-ogy (eg, stroke, head injury, trauma). Once it is
determined that the patient is a candidate for t-PA therapy, no anticoagulants
are to be administered in the next 24 hours.
Before
receiving t-PA, the patient should be assessed using the National Institutes of
Health Stroke Scale (NIHSS), which con-tains 42 items evaluating neurologic
deficits and is useful in dif-ferentiating between ischemic strokes and TIAs
(Table 62-4). A patient with an NIHSS score of greater than 22 is not eligible
to receive t-PA.

Dosage and Administration.
The patient is weighed to deter-mine the dose of t-PA. The minimum dose is 0.9 mg/kg; the maximum dose is 90 mg. The loading dose is 10% of the calcu-lated dose and is administered over 1 minute. The remaining dose is administered over 1 hour via an infusion pump. After the in-fusion is completed, the line is flushed with 20 mL of normal saline solution to ensure that all the medication is administered.
The
patient is admitted to the intensive care unit, where con-tinuous cardiac
monitoring is implemented. Vital signs are ob-tained every 15 minutes for the
first 2 hours, every 30 minutes for the next 6 hours, then every hour for 16
hours. Blood pressure should be maintained with the systolic pressure less than
180 mm Hg and the diastolic pressure less than 100 mm Hg. Airway man-agement is
instituted based on the patient’s clinical condition and arterial blood gas
values.
Side Effects.
Bleeding is the most common side effect of t-PA
ad-ministration, and the patient should be closely monitored for any bleeding
(intracranial, intravenous [IV] insertion sites, urinary catheter site,
endotracheal tube, nasogastric tube, urine, stool, emesis, other secretions)
(Scroggins, 2000). Intracranial bleeding is a major complication that occurs in
approximately 6.5% of pa-tients (NINDS t-PA Stroke Study Group, 1995).
THERAPY FOR PATIENTS WITH ISCHEMIC STROKE NOT RECEIVING t-PA
Not
all patients are candidates for t-PA therapy. Other treatments include
anticoagulant administration (IV heparin or low-molecular-weight heparin) for
ischemic strokes and careful maintenance of cerebral hemodynamics to maintain
cerebral perfusion. Increased
intracranial pressure (ICP) and its associated complications may occur
following a large ischemic stroke. Interventions during this period include
methods to reduce ICP, such as administer-ing an osmotic diuretic (eg,
mannitol), maintaining PaCO2 within the range of 30 to 35 mm Hg, and
positioning to avoid hy-poxia. Other treatment measures include the following:
·
Elevation of the head of the
bed to promote venous drainage and to lower increased ICP
·
Intubation with an
endotracheal tube to establish a patent airway, if necessary
·
Continuous hemodynamic
monitoring. Systolic pressure should be maintained at less than 180 mm Hg,
diastolic pressure at less than 100 mm Hg. Maintaining the blood pressure
within this range reduces the potential for addi-tional bleeding or further
ischemic damage.Neurologic assessment to determine whether the stroke is
evolving or whether other acute complications are develop-ing, such as bleeding
from anticoagulation or medication-induced bradycardia, which can result in
hypotension and subsequent decreases in cardiac output and cerebral perfu-sion
pressure.
See the acute ischemic stroke clinical guidelines
in Appendix A.
MANAGING POTENTIAL COMPLICATIONS
Adequate
cerebral blood flow is essential for cerebral oxygenation. If cerebral blood
flow is inadequate, the amount of oxygen sup-plied to the brain will decrease
and tissue ischemia will result. Therefore, maintaining cardiac output within
the normal range of 4 to 8 L/min, or sometimes greater, can improve the
cerebral blood flow and oxygen delivery. Adequate oxygenation begins with
pulmonary care, maintenance of a patent airway, and ad-ministration of
supplemental oxygen as needed. The importance of adequate gas exchange cannot
be overemphasized in these pa-tients, many of whom are elderly and more prone
to developing pneumonia, which can interfere with gas exchange.
ENDARTERECTOMY FOR PREVENTION OF ISCHEMIC STROKE
The main surgical procedure for managing TIAs and
small stroke is carotid endarterectomy, currently the most frequently
per-formed peripheral vascular procedure in the United States (Krenzer, 1999).
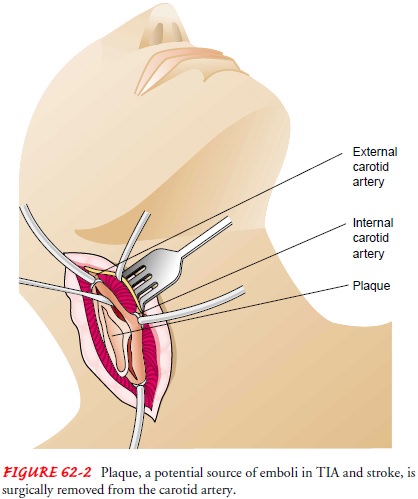
A carotid endarterectomy is the removal of an atherosclerotic plaque or
thrombus from the carotid artery to pre-vent stroke in patients with occlusive
disease of the extracranial cerebral arteries (Fig. 62-2). This surgery is
indicated for patients with symptoms of TIA or mild stroke found to be due to
severe (70% to 99%) carotid artery stenosis or moderate (50% to 69%) stenosis
with other significant risk factors (Wolf et al., 1999).
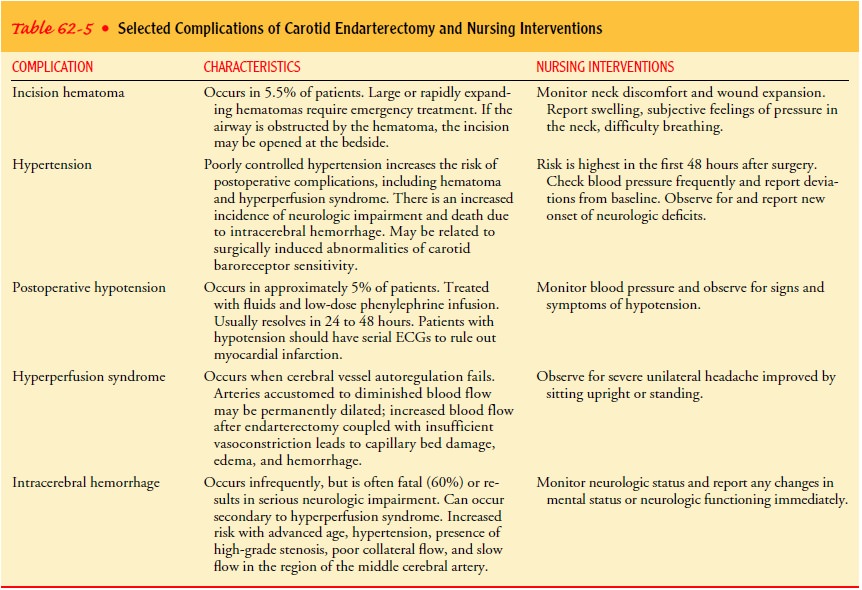
Nursing Management.
The primary complications of carotid
en-darterectomy are stroke, cranial nerve injuries, infection or hematoma at
the incision, and carotid artery disruption. It is im-portant to maintain
adequate blood pressure levels in the imme-diate postoperative period.
Hypotension is avoided to prevent cerebral ischemia and thrombosis. Uncontrolled
hypertension may precipitate cerebral hemorrhage, edema, hemorrhage at the
surgical incision, or disruption of the arterial reconstruction. Sodium
nitroprusside is commonly used to reduce the blood pres-sure to previous
levels. Close cardiac monitoring is necessary be-cause these patients have a
high incidence of coronary artery disease.
A neurologic flow sheet is used to monitor and
document all body systems, with particular attention to neurologic status,
fol-lowing carotid endarterectomy. The neurosurgeon is notified immediately if
a neurologic deficit develops. Formation of a thrombus at the site of the
endarterectomy is suspected if there is a sudden increase in neurologic
deficits, such as weakness on one side of the body. The patient should be prepared
for repeat endarterectomy.
Difficulty in swallowing, hoarseness, or other
signs of cranial nerve dysfunction must be assessed. The nurse should focus on
assessment of cranial nerves VI, X, XI, and XII (Krenzer, 1999). Some swelling
in the neck after surgery is expected; if large enough, however, swelling and
hematoma formation can obstruct the airway. Emergency airway supplies,
including those needed for a tracheostomy, must be available. Table 62-5
provides more information about potential complications of carotid surgery.
Related Topics