Chapter: Medical Surgical Nursing: Management of Patients With Cerebrovascular Disorders
Hemorrhagic Stroke
Hemorrhagic Stroke
Hemorrhagic strokes account for 15% of
cerebrovascular disor-ders and are primarily caused by an intracranial or
subarachnoid hemorrhage. Each year in the United States there are
approxi-mately 50,000 intracerebral hemorrhages and 25,000 cases of subarachnoid
hemorrhage from ruptured intracranial aneurysm
(Pfohman & Criddle, 2001; Qureshi et al., 2001).
Patients generally have more severe deficits and a
longer re-covery time compared to those with ischemic stroke (AHCPR, 1995). The
mean cost per discharge for subarachnoid hemor-rhage was estimated at $39,994,
compared to $21,535 for in-tracranial hemorrhage. The mean length of stay was
22 days for subarachnoid hemorrhage and 19 for intracranial hemorrhage (Matchar
& Samsa, 2000).
Hemorrhagic strokes are caused by bleeding into the
brain tis-sue, the ventricles, or the subarachnoid space. Primary
intracere-bral hemorrhage from a spontaneous rupture of small vessels accounts
for approximately 80% of hemorrhagic strokes and is primarily caused by uncontrolled
hypertension (Qureshi et al., 2001). Secondary intracerebral hemorrhage is
associated with arteriovenous malformations (AVMs), intracranial aneurysms, or
certain medications (eg, anticoagulants and amphetamines) (Qureshi et al.,
2001).
Pathophysiology
The pathophysiology of hemorrhagic stroke depends
on the cause and type of cerebrovascular disorder. Symptoms are produced when
an aneurysm or AVM enlarges and presses on nearby cranial nerves or brain
tissue or, more dramatically, when ananeurysm or AVM ruptures,
causing subarachnoid hemorrhage (hemorrhage into the cranial subarachnoid
space). Normal brain metabolism is disrupted by the brain being exposed to
blood; by an increase in ICP resulting from the sudden entry of blood into the
subarachnoid space, which compresses and injures brain tissue; or by secondary
ischemia of the brain resulting from the reduced perfusion pressure and
vasospasm that frequently accompany subarachnoid hemorrhage.
INTRACEREBRAL HEMORRHAGE
An intracerebral hemorrhage, or bleeding into the
brain sub-stance, is most common in patients with hypertension and cere-bral
atherosclerosis because degenerative changes from these diseases cause rupture
of the vessel. They also may be due to cer-tain types of arterial pathology,
brain tumor, and the use of med-ications (oral anticoagulants, amphetamines,
and illicit drugs such as crack and cocaine).
The bleeding is usually arterial and occurs most
commonly in the cerebral lobes, basal ganglia, thalamus, brain stem (mostly the
pons), and cerebellum (Qureshi et al., 2001). Occasionally, the bleeding
ruptures the wall of the lateral ventricle and causes in-traventricular
hemorrhage, which is frequently fatal.
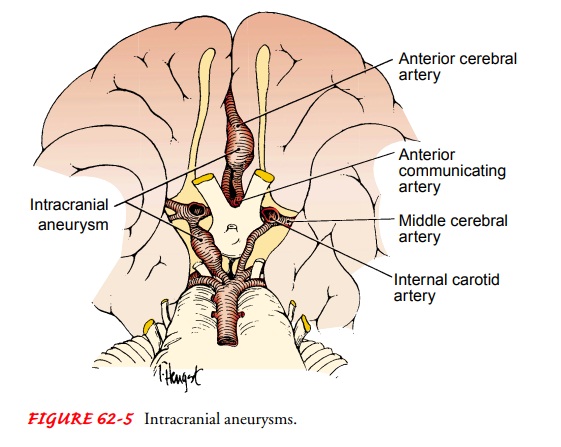
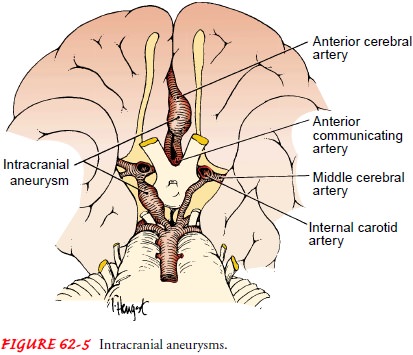
INTRACRANIAL (CEREBRAL) ANEURYSM
An intracranial (cerebral) aneurysm is a dilation of the walls of a cerebral artery that
develops as a result of weakness in the arterial wall. The cause of aneurysms
is unknown, although research is ongoing. An aneurysm may be due to
atherosclerosis, resulting in a defect in the vessel wall with subsequent
weakness of the wall; a congenital defect of the vessel wall; hypertensive
vascular dis-ease; head trauma; or advancing age.
Any artery within the brain can be the site of
cerebral aneurysms, but they usually occur at the bifurcations of the large arteries
at the circle of Willis (Fig. 62-5). The cerebral arteries most com-monly
affected by an aneurysm are the internal carotid artery (ICA), anterior
cerebral artery (ACA), anterior communicating artery (ACoA), posterior
communicating artery (PCoA), poste-rior cerebral artery (PCA), and middle
cerebral artery (MCA). Multiple cerebral aneurysms are not uncommon.
ARTERIOVENOUS MALFORMATIONS
An
AVM is due to an abnormality in embryonal development that leads to a tangle of
arteries and veins in the brain without a
SUBARACHNOID HEMORRHAGE
A subarachnoid hemorrhage (hemorrhage into the
subarachnoid space) may occur as a result of an AVM, intracranial aneurysm,
trauma, or hypertension. The most common cause is a leaking aneurysm in the
area of the circle of Willis or a congenital AVM of the brain.
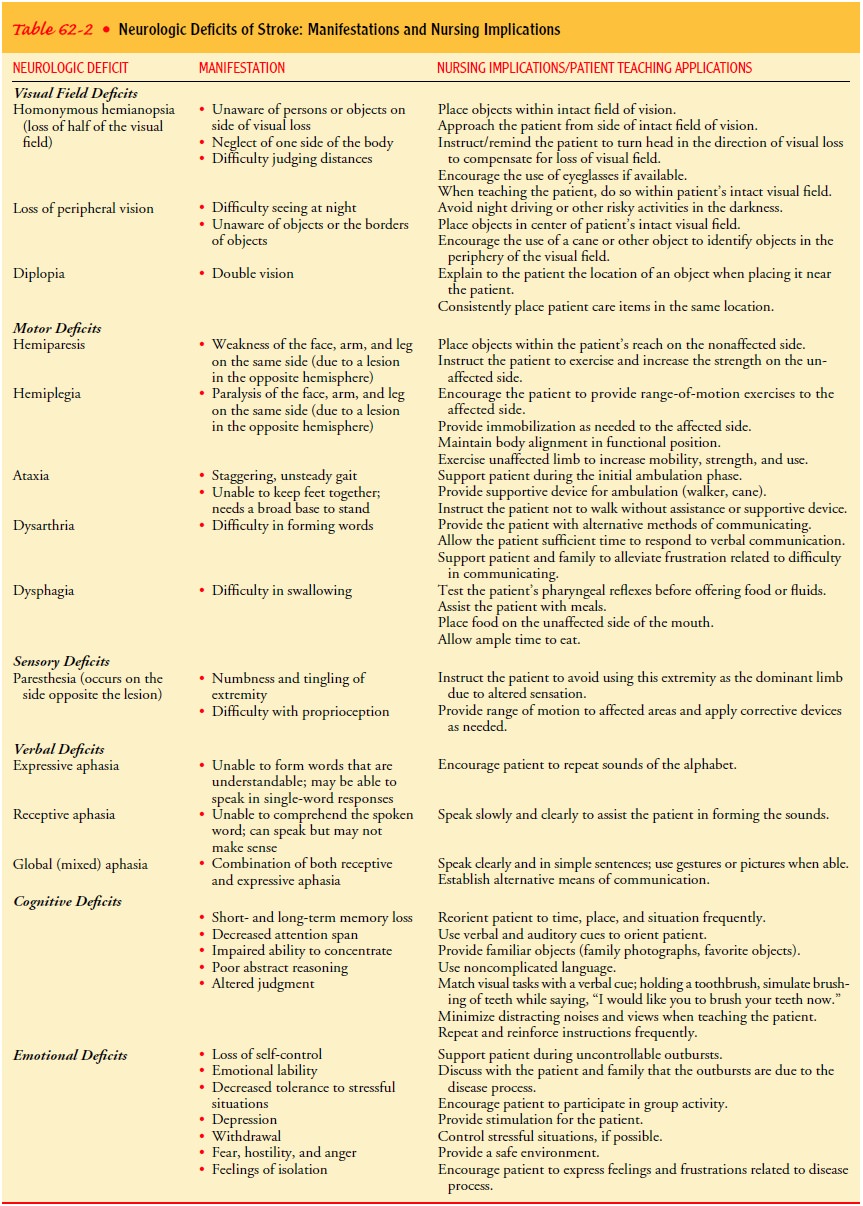
Clinical Manifestations
The patient with a
hemorrhagic stroke can present with a wide variety of neurologic deficits,
similar to the patient with ischemic stroke. A comprehensive assessment will
reveal the extent of the neurologic deficits. Many of the same motor, sensory,
cranial nerve, cognitive, and other functions that are disrupted following
ischemic stroke are altered following a hemorrhagic stroke. Table 62-2 reviews
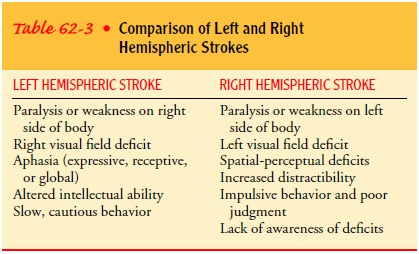
the neurologic deficits frequently seen in stroke pa-tients. Table 62-3
compares the symptoms seen in right hemi-spheric stroke with those seen in left
hemispheric stroke.
In addition to the neurologic deficits that are similar
to is-chemic stroke, the patient with an intracranial aneurysm or AVM can have
some unique clinical manifestations. Rupture of an aneurysm or AVM usually
produces a sudden, unusually severe headache and often loss of consciousness
for a variable period. There may be pain and rigidity of the back of the neck
(nuchal rigidity) and spine due to meningeal irritation. Visual distur-bances
(visual loss, diplopia, ptosis) occur when the aneurysm is adjacent to the
oculomotor nerve. Tinnitus, dizziness, and hemi-paresis may also occur.
At times, an aneurysm or AVM leaks blood, leading to the
for-mation of a clot that seals the site of rupture. In this instance, the
patient may show little neurologic deficit. In other cases, severe bleeding
occurs, resulting in cerebral damage followed rapidly by coma and death.
Prognosis depends on the neurologic condition of the
patient, age, associated diseases, and the extent and location of an
intra-cranial aneurysm. Subarachnoid hemorrhage from an aneurysm is a
catastrophic event with significant morbidity and mortality (Pfohman &
Criddle, 2001). Chart 62-6 discusses ethical issues related to the patient with
a severe hemorrhagic stroke.

Assessment and Diagnostic Findings
Any patient suspected of having a hemorrhagic stroke
should un-dergo CT scanning to determine the size and location of the hematoma
as well as the presence or absence of ventricular blood and hydrocephalus
(Qureshi et al., 2001). CT scan and cerebral angiography confirm the diagnosis
of an intracranial aneurysm or AVM. These tests show the location and size of
the lesion and provide information about the affected arteries, veins,
adjoining vessels, and vascular branches. Lumbar puncture is performed if there
is no evidence of increased ICP, the CT scan results are neg-ative, and
subarachnoid hemorrhage must be confirmed. Lumbar puncture in the presence of
increased ICP could result in brain stem herniation or rebleeding. In
diagnosing a hemorrhagic stroke in a patient younger than 40, some clinicians
obtain a tox-icology screen for illicit drug use.
The Hunt-Hess classification system guides the physician in diagnosing the severity of subarachnoid hemorrhage after an aneurysmal bleed (Table 62-6). Classifying the patient by severity of neurologic deficit provides a baseline for future comparison.
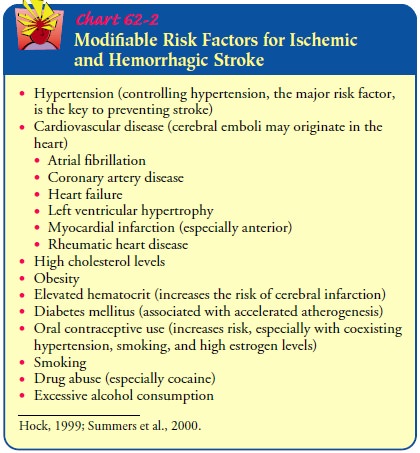
Prevention
Primary prevention of hemorrhagic stroke is the best
approach and includes managing hypertension and ameliorating other sig-nificant
risk factors (Pfohman & Criddle, 2001). Control of hy-pertension,
especially in individuals over 55 years of age, clearly reduces the risk for
hemorrhagic stroke (Qureshi et al., 2001). Additional factors are similar to
the risks for ischemic stroke and include smoking, excessive alcohol intake,
and high cholesterol (see Chart 62-2). Stroke risk screenings provide an ideal
oppor-tunity to lower hemorrhagic stroke risk by identifying high-risk
individuals or groups and educating the patients and the com-munity about
recognition and prevention (Pfohman & Criddle, 2001).
A prevention effort unique to hemorrhagic stroke is to
increase the public’s awareness about the association between phenyl-propanolamine
(an ingredient found in appetite suppressants as well as cold and cough agents)
and hemorrhagic stroke. Recent research has found that phenylpropanolamine is
an independent risk factor for hemorrhagic stroke, especially in women (Kernan
et al., 2000). Many products have been removed voluntarily from the market, but
consumers should continue to look for this in-gredient on labels.
Medical Management
The goals of medical
treatment of hemorrhagic stroke are to allow the brain to recover from the initial
insult (bleeding), to prevent or minimize the risk for rebleeding, and to
prevent or treat com-plications. Management consists of bed rest with sedation
to pre-vent agitation and stress, management of vasospasm, and surgical or
medical treatment to prevent rebleeding. Analgesics (codeine, acetaminophen)
may be prescribed for head and neck pain. The patient is fitted with elastic
compression stockings to prevent deep vein thrombosis, a threat to any patient
on bed rest.
COMPLICATIONS
Potential complications include rebleeding; cerebral
vasospasm resulting in cerebral ischemia; acute hydrocephalus, which results
when free blood obstructs the reabsorption of cerebrospinal fluid (CSF) by the
arachnoid villi; and seizures.
Cerebral Hypoxia and Decreased Blood Flow.
Immediate
com-plications of a hemorrhagic stroke include cerebral hypoxia, de-creased
cerebral blood flow, and extension of the area of injury. Providing adequate
oxygenation of blood to the brain minimizes cerebral hypoxia. Brain function is
dependent on available oxy-gen being delivered to the tissues. Administering
supplemental oxygen and maintaining the hemoglobin and hematocrit at
ac-ceptable levels will assist in maintaining tissue oxygenation.
Cerebral blood flow is dependent on the blood pressure,
car-diac output, and integrity of cerebral blood vessels. Adequate hy-dration
(IV fluids) must be ensured to reduce blood viscosity and improve cerebral
blood flow. Extremes of hypertension or hy-potension need to be avoided to
prevent changes in cerebral blood flow and the potential for extending the area
of injury.
A seizure can also compromise cerebral blood flow.
Seizures occur in approximately 5% of stroke patients (Berges et al., 2000).
Observation for and appropriate treatment of seizure ac-tivity is an important
component of care following a hemorrhagic stroke (Qureshi et al., 2001).
Vasospasm.
The development of cerebral vasospasm (narrowingof the lumen of the
involved cranial blood vessel) is a serious com-plication of subarachnoid
hemorrhage and accounts for 40% to 50% of the morbidity and mortality of those
who survive the ini-tial intracranial bleed. The mechanism responsible for the
spasm is not clear, but vasospasm is associated with increasing amounts of
blood in the subarachnoid cisterns and cerebral fissures, as visualized by CT
scan.
Vasospasm leads to increased vascular resistance, which
im-pedes cerebral blood flow and causes brain ischemia and infarc-tion. The
signs and symptoms reflect the areas of the brain involved. Vasospasm is often
heralded by a worsening headache, a decrease in level of consciousness
(confusion, lethargy, and disorientation), or a new focal neurologic deficit
(aphasia, hemi-paresis [partial paralysis affecting one side of the body]).
Vasospasm frequently occurs 4 to 14 days after initial
hemor-rhage when the clot undergoes lysis (dissolution), increasing the chances
of rebleeding.
It is believed that
early surgery to clip the aneurysm prevents rebleeding and that removal of
blood from the basal cisterns around the major cerebral arteries may prevent
vasospasm. The IV administration of the calcium-channel blocker nimodipine
during the critical time in which vasospasm may occur may pre-vent delayed
ischemic deterioration. Advances in technology have led to the introduction of
interventional neuroradiology for the treatment of aneurysms. Endovascular
techniques may be used in selected patients to occlude the artery supplying the
aneurysm with a balloon or to occlude the aneurysm itself. As more studies on
these techniques are completed, their use will increase.
Management of vasospasm
remains difficult and controver-sial. Based on one theory that vasospasm is
caused by an increased influx of calcium into the cell, medication therapy may
be used to block or antagonize this action and prevent or reverse the ac-tion
of vasospasm already present. Calcium-channel blockers may include nimodipine
(Nimotop), verapamil (Isoptin), and nifedi-pine (Procardia). Other therapy for
vasospasm is aimed at mini-mizing the deleterious effects of the associated
cerebral ischemia and includes fluid volume expanders and induced arterial
hyper-tension, normotension, or hemodilution.
Increased ICP.
An increase in ICP can follow either an ischemicor
hemorrhagic stroke but almost always follows a subarachnoid hemorrhage, usually
because of disturbed circulation of CSF caused by blood in the basal cisterns.
If the patient shows evi-dence of deterioration from increased ICP (due to
cerebral edema, herniation, hydrocephalus, or vasospasm), CSF drainage may be
instituted by cautious lumbar puncture or ventricular catheter drainage, and
mannitol is given to reduce ICP. When mannitol is used as a long-term measure
to control ICP, dehy-dration and disturbances in electrolyte balance
(hyponatremia or hypernatremia; hypokalemia or hyperkalemia) may occur.
Man-nitol acts by pulling water out of the brain tissue by osmosis as well as
by reducing total-body water through diuresis. The pa-tient is monitored for
signs of dehydration and for rebound ele-vation of ICP.
Systemic Hypertension.
Preventing sudden
systemic hyperten-sion is critical in hemorrhagic stroke management. The goal
of therapy is to maintain the systolic blood pressure at about 150 mm Hg. If
blood pressure is elevated, antihypertensive therapy (labetalol [Normodyne],
nicardipine [Cardene], nitroprusside [Nitropress]) may be prescribed.
Hemodynamic monitoring by arterial line during the administration of
antihypertensives is im-portant to detect and avoid a precipitous drop in blood
pressure, which can produce brain ischemia. Because seizures cause blood
pressure elevation, antiseizure agents are administered prophy-lactically.
Stool softeners are used to prevent straining, which can also elevate the blood
pressure.
SURGICAL MANAGEMENT
Many patients with a
primary intracerebral hemorrhage are not treated surgically. However, surgical
evacuation is strongly rec-ommended for the patient with a cerebellar
hemorrhage if the di-ameter exceeds 3 cm and the Glasgow Coma Scale score is
below 14 (Qureshi et al., 2001). Surgical evacuation is most frequently
accomplished via a craniotomy.
The patient with an
intracranial aneurysm is prepared for sur-gical intervention as soon as the
condition is considered stable. The Hunt-Hess classification system guides the
physician in diagnosing the severity of subarachnoid hemorrhage after an
aneurysm bleeds and in timing the surgery (see Table 62-6). Mor-bidity and
mortality from surgery are high if the patient is stu-porous or comatose (grade
IV or V). Surgical treatment of the patient with an unruptured aneurysm is an
option (Pfohman & Criddle, 2001).
The goal of surgery is
to prevent bleeding in an unruptured aneurysm and further bleeding in an
already ruptured aneurysm. This objective is accomplished by isolating the
aneurysm from its circulation or by strengthening the arterial wall. An
aneurysm may be excluded from the cerebral circulation by means of a lig-ature
or a clip across its neck. If this is not anatomically possible, the aneurysm
can be reinforced by wrapping it with muslin or some other substance to provide
support and induce scarring.
An
extracranial-intracranial arterial bypass may be performed to establish
collateral blood supply to allow surgery on the aneurysm. Alternatively, an
extracranial method may be used, whereby the carotid artery is gradually
occluded in the neck to re-duce pressure within the blood vessel. After
ligation of the carotid artery, there is some risk for cerebral ischemia and
sudden hemi-plegia because during the surgical procedure, there is a temporary
occlusion of the blood supply to the brain (unless a temporary by-pass shunt is
used). In anticipation of these complications, cere-bral blood flow and
internal carotid pressure may be measured to identify patients at risk for
postoperative ischemic episodes.
Several less invasive
endovascular treatments are now being used for aneurysms. These procedures are
performed by neuro-surgeons in neurointerventional radiology suites. Two
procedures are endovascular treatment (occlusion of the parent artery) and
aneurysm coiling (obstruction of the aneurysm site with a coil). While
associated with lower risks than intracranial surgery in gen-eral, secondary
stroke and rupture of the aneurysm are still po-tential complications (Pfohman
& Criddle, 2001).
Postoperative
complications include psychological symptoms (disorientation, amnesia, Korsakoff ’s syndrome, personality
changes), intraoperative embolization, postoperative internal artery occlusion,
fluid and electrolyte disturbances (from dys-function of the neurohypophyseal
system), and gastrointestinal bleeding.
Related Topics