Chapter: High Voltage Engineering : Electrical breakdown is gases, solids& Liquids
Ionization and Decay Processes
IONIZATION AND DECAY PROCESSES
At normal
temperature and pressure gases are excellent insulators. The conduction in air at
low field is in the region 10-16 ŌĆō 10-17 A/cm2. These
current results from cosmic radiations and radioactive substances present in
earth and the atmosphere. At higher fields charged particles may gain
sufficient energy between collisions to cause ionization on impact with neutral
molecules.
It was
shown in the previous section that electrons on average lose little energy in
elastic collisions and readily build up their kinetic energy which may be
supplied by an external source, e.g. an applied field. On the other hand,
during inelastic collisions a large fraction of their kinetic energy is
transferred into potential energy, causing, for example, ionization of the
struck molecule. Ionization by electron impact is for higher field strength the
most important process leading to breakdown of gases. The effectiveness of ionization
by electron impact depends upon the energy that an electron can gain along the
mean free path in the direction of the field.
This
simple model is not applicable for quantitative calculations, because
ionization by collision, as are all other processes in gas discharges, is a
probability phenomenon, and is generally expressed in terms of cross-section
for ionization defined as the product PiŽā = Žāi where Pi is the probability of
ionization on impact and Žā is the molecular or atomic cross-sectional area for
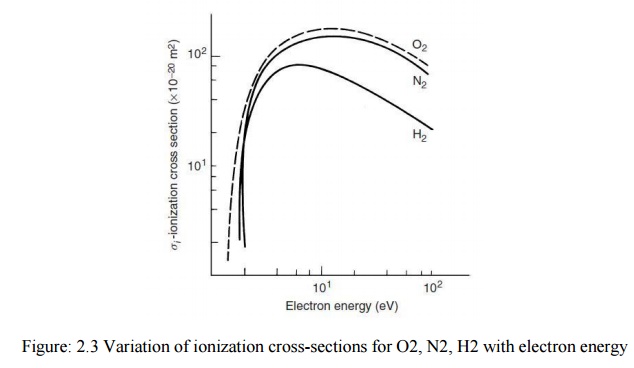
interception defined earlier. The cross-section &i is measured using
monoenergetic electron
beams of
different energy. The variation of ionization cross-sections for H2,
O2, and N2 with electron energy.
It is
seen that the cross-section is strongly dependent upon the electron energy. At
energies below ionization potential the collision may lead to excitation of the
struck atom or molecule which on collision with another slow moving electron
may become ionized. This process becomes significant only when densities of
electrons are high. Very fast moving electrons may pass near an atom without
ejecting an electron from it. For every gas there exists an optimum electron
energy range which gives a maximum ionization probability.
Related Topics