Chapter: Obstetrics and Gynecology: Fetal Growth Abnormalities:Intrauterine Growth Restriction and Macrosomia
Intrauterine Growth Restriction
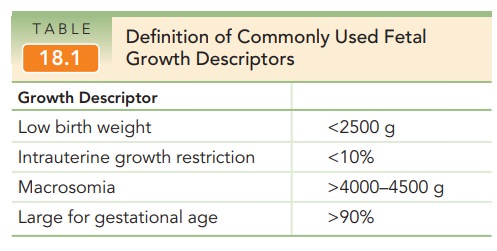
INTRAUTERINE GROWTH RESTRICTION
“Fetal growth restriction”
describes infants whose weights are much lower than expected. Population-based
norms are used to categorize abnormal growth. A fetus or infantwhose weight is less than the 10th percentile of a
specific pop-ulation at a given gestational age is designated as having intrauterine growth restriction [IUGR](Table
18.1).Therefore, careful assignment of gestational age is cru-cial to the
diagnosis and management of patients with IUGR.
The term “small for gestational age” (SGA) is used to describe an infant
with a birth weight at the lower extreme of the normal birth weight
distribution. In the United States, the most commonly used definition of SGA is
a birth weight below the 10th percentile for gestational age. The use of the
terms “small for gestational age” (SGA) and “intrauterine growth restriction”
has been confusing, and the terms often are used interchangeably.
The use of gestational age
percentiles remains lim-ited for a number of reasons. First, by definition, the
preva-lence of IUGR will be 10%, but not all such neonates are pathologically
small. Second, any percentile cut-off fails to take into account an
individual’s growth potential. Also, a simple percentile cannot take into
account growth rate. The change in percentile over time or change in specific
measurements may be more important. Finally, the time when the growth
restriction is found may be a factor in morbidity and mortality: growth
restriction at earlier gestational ages has greater effects on morbidity and
mortality.
Significance
The goal
of recognizing neonates with growth abnormalities is to identify infants at
risk for increased short-term and long-term morbidity or mortality.
In the short-term, the growth
restricted fetus potentially lacks adequate reserves to continue intrauterine
existence, to undergo the stress of labor, or to fully adapt to neonatal life.
These conditions make the infant vulnerable to intra-uterine fetal death,
asphyxia, acidemia, and intolerance to labor. Neonatal complications, low Apgar
scores, poly-cythemia, hyperbilirubinemia, hypoglycemia, hypothermia, apnea,
respiratory distress, seizures, sepsis, meconium aspi-ration, and neonatal
death.
Alterations in fetal growth may
have lifelong implica-tions. The antenatal response or fetal adaptation to the
intrauterine nutritional and metabolic environment may predict or dictate the
response to an extrauterine environ-ment. Increasing evidence supports the
concept of fetal origins for adult diseases and the association between birth
size and long-term health. Associations have been reported between birth weight
and adult obesity, cardiovascular dis-ease (coronary heart disease,
hypertension, and stroke), insulin resistance, and dyslipidemia. Therefore,
intrauter-ine growth may reflect the foundation of many aspects of lifelong
physiologic function.
In general, the smaller the fetus with IUGR, the greater its risk for morbidity and mortality. Perinatal morbidity andmortality is significantly increased in the presence of low birth weight for gestational age, especially with weights below the 3rd percentile for gestational age. One study found that 26% of all stillbirths were SGA. Thus, it is important to identify such infants in utero so that management maximizes the quality of their intrauterine environment, permits planning and implementation of delivery using the safest means possible, and provides necessary care in the neonatal period.
Pathophysiology
For a fetus to thrive in utero,
an adequate number of fetal cells and cells that differentiate properly are
both requisite. In addition, nutrients and oxygen must be available via an
adequately functioning uteroplacental unit to allow an increase in the number
of cells and in cell size. Early in pregnancy, fetal growth occurs primarily
through cellularhyperplasia, or cell
division, and early-onset IUGR maylead to an irreversible diminution of organ
size and, per-haps, function. Early-onset IUGR is also more commonly associated
with heritable factors, immunologic abnormal-ities, chronic maternal disease,
fetal infection, and multi-ple pregnancies. Later in pregnancy, fetal growth
depends increasingly on cellular
hypertrophy rather than hyper-plasia alone, so that delayed-onset IUGR may
also result in decreased cell size, which may be more amenable to restoration
of fetal size with adequate nutrition. The nor-mal fetus grows throughout the
pregnancy, but the rate of growth decreases after 37 weeks of gestational age
as the fetus depletes fat for cellular growth.
The placenta grows early and rapidly compared with the fetus, reaching
a maximum surface area of about 11 m2 and weight of 500 g at
approximately 37 weeks of gesta-tional age. Thereafter, there is a slow but
steady decline in placental surface area (and, hence, function), primarily
because of microinfarctions of its vascular system. Late-onset growth
restriction may therefore be primarily related to decreased function and
nutrient transport of the utero-placental unit, a condition termed uteroplacental insuf-ficiency. In
addition, because there is a close relationshipbetween placental surface area
and fetal weight, factors that act to decrease placental size are also
associated with decreased (i.e., restricted) growth.
Etiology
IUGR is a
descriptive term for a condition that has numerous potential causes. Determining
the specific diagnosis is impor-tant for optimal management. Although a number
of causes of IUGR have been recognized, a definite etiology of IUGR cannot be
identified in approximately 50% of all cases. In addition, because the
utilization of a percentile cut-off of 10% alone will result in a high
proportion of false-positives, two-thirds or more of such fetuses categorized
as IUGR will be simply constitutionally small and otherwise healthy.
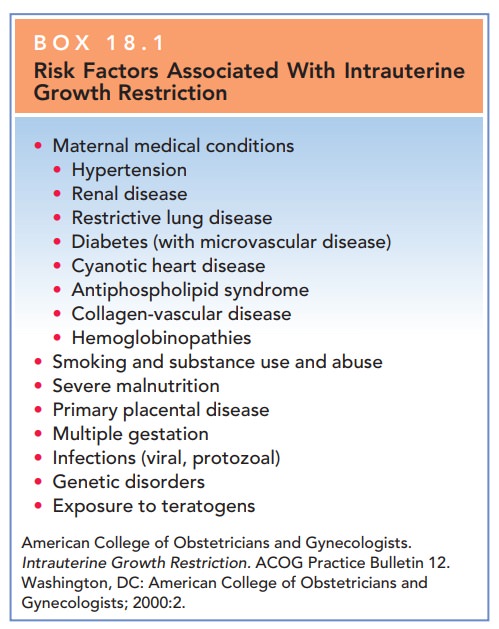
Factors that affect fetal growth
are extensive and include maternal, fetal, and placental causes; these are
listed in Box 18.1.
MATERNAL FACTORS
Maternal factors include viral
infections, such as rubella, varicella, and cytomegalovirus, which are
associated with high rates of growth restriction, particularly if infection
occurs early in pregnancy. Although these infections may manifest only as mild
“flu-like” illnesses, injury to the fetus during organogenesis can result in a
decreased cell number, resulting in diminished growth with or without multiple
congenital anomalies. Five percent or fewer of all cases of IUGR are related to
early infection with these or other viral agents. Maternal substance abuse
affects fetal growth and almost all infants with fetal alcohol syn-drome will
be growth-restricted. Women who smoke during pregnancy deliver babies 200 g
smaller on average than do women who do not smoke; moreover, the rate of growth
restriction is 3- to 4-fold greater among babies born to women who smoke during
pregnancy. Women who use narcotics, heroin, methadone, or cocaine also have
rates of growth-restricted babies ranging from as much as 30% to 50%.
Medications known to be associ-ated with IUGR include anticonvulsant
medications, warfarin, and folic acid antagonists. Altitude may also affect
fetal growth.
Other maternal factors that
affect fetal growth and body composition include demographic factors and
med-ical conditions. Extremes in maternal age (age younger than 16 years and
older than 35 years) are associated with an increased risk of fetal growth
restriction. Medical conditions that alter or affect placental function may
also be causative factors.
Although one common pathway has
not been clearly identified, many of these disorders occur together. Women with
a history of prior obstetric complications have an increased risk of growth
abnormalities. Maternal metabo-lism and body composition are two of the
strongest regula-tors of fetal growth. Nutritional deficiencies and inadequate
weight gain, particularly in teens or in underweight women, may result in IUGR.
FETAL FACTORS
The inherent growth potential of
the individual is deter-mined genetically. Female fetuses are at greater risk
for IUGR than males. In addition, up to 20% of growth-restricted fetuses have a
chromosomal abnormality. In addition, single-gene mutations such as the
glucokinase gene mutation, or genetic syndromes such as Beckwith-Wiedemann
syndrome can also result in abnormalities of growth. Finally, multifetal
pregnancies are at increased risk for growth restriction.
PLACENTAL FACTORS
The placenta is critical for
nutrient regulation and trans-portation from mother to fetus. Abnormalities in
placenta-tion or defective trophoblast invasion and remodeling may contribute
to fetal growth restriction as well as other dis-orders of pregnancy. In
addition, uterine anomalies (uter-ine septum or fibroids) may limit placental
implantation and development and, consequently, nutrient transport, result-ing
in inadequate nutrition for the developing fetus. Finally, the genetic
composition of the placenta is important and abnormalities such as confined
placental mosaicism are asso-ciated with growth delay.
Diagnosis
Assessment of gestational age is
important in early preg-nancy, because dating becomes increasingly imprecise at
later gestational ages.Antenatal
recognition of IUGR depends upon the recognition of risk factors and the
clinical assessment of uterine size, fol-lowed by biometric measurements.
Physical examination is limited
in usefulness in recognizing IUGR or in making a specific diagnosis, but it is
an impor-tant screening test for abnormal fetal growth. Maternal size and
weight gain throughout pregnancy also have limited value, but access to such
information is readily available; a low maternal weight or little or no weight
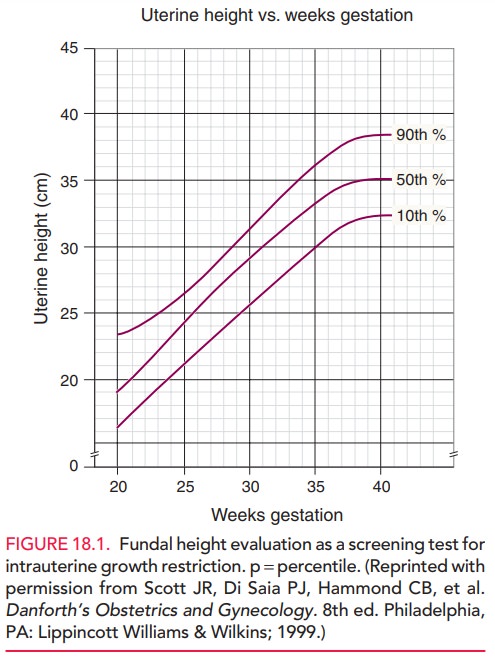
gain during pregnancy may suggest IUGR. Serial measurements of fundal height are commonly used as a
screening test forIUGR, but have high rates of false-negative and
false-positive predictive values. Between 20 and 36 weeks of ges-tation, fundal
height should increase approximately 1 cm per week, consistent with gestational
age in weeks (Fig. 18.1). A discrepancy may be related to constitutional
factors, but a significant discrepancy of more than 2 cm may indicate IUGR and
the need for an ultrasound examination. Clinical estimations of fetal weight
alone are not helpful in diagnos-ing IUGR, except when fetal size is grossly
diminished.
If IUGR
is suspected based on risk factors and clinical assessment, ultrasonography
should be performed to assess fetal size and growth. Specificfetal biometry measurementsare compared
with standardized tables that reflect normal growth at a certain gestational
age. The four standard fetal measurements include the (1) biparietal diameter,
(2) head circumference (HC), (3) abdominal circumference (AC), and (4) femur
length. Conversion of individual morphologic measurements to fetal weight using
published equations or ratios of measurements can provide useful estimations of
fetal size. An abdominal circumference within the normal range reliably
excludes growth restriction, with a false-negative rate of less than 10%. A
small abdominal circum-ference or fetal weight estimate below the 10th
percentile suggests the possibility of growth restriction, with the likelihood
increasing as the percentile rank decreases.
When IUGR
is suspected, serial measurements of fetal biometric parameters provide an
estimated growth rate. Suchserial measurements are of
considerable clinical value in confirming or excluding the diagnosis and
assessing the progression and severity of growth restriction. Given the high
incidence of genetic and structural defects associated with IUGR, a detailed
ultrasound survey for the presence of fetal structural and functional defects
may be indicated.
Following
recognition of altered fetal growth, a search for potential etiology should
ensue. Ultrasonography should in-clude a detailed anatomic
survey to evaluate for the pres-ence of structural anomalies, given the high
incidence of genetic and structural defects with IUGR. Ultrasound eval-uation
should also include an assessment of amniotic
fluidvolume. The combination of oligohydramnios (dimin-ished amniotic fluid
volume) and IUGR is associated with severe disease and increased morbidity. The
mechanism of decreased amniotic fluid is thought to be decreased placen-tal
perfusion of oxygen and nutrients with a compensatory redistribution of fetal
blood favoring the brain, adrenal gland, and heart. The consequent decrease in
fetal blood to the kidneys leads to a reduction of urine output, which is the
primary source of amniotic fluid in the second half of pregnancy.
Direct invasive studies of the
fetus are useful in selected patients with IUGR. Amniocentesis for fetal lung
matu-rity may assist delivery planning near term or when there is uncertainty
regarding gestational age and concern for growth restriction. Fetal karyotyping
and viral cultures and polymerase chain reactions can be performed on fluid
obtained by amniocentesis. Rarely, chorionic
villus sam-pling (biopsy of placenta) or direct blood sampling (per-cutaneous umbilical blood sampling)
may be necessaryfor specific studies.
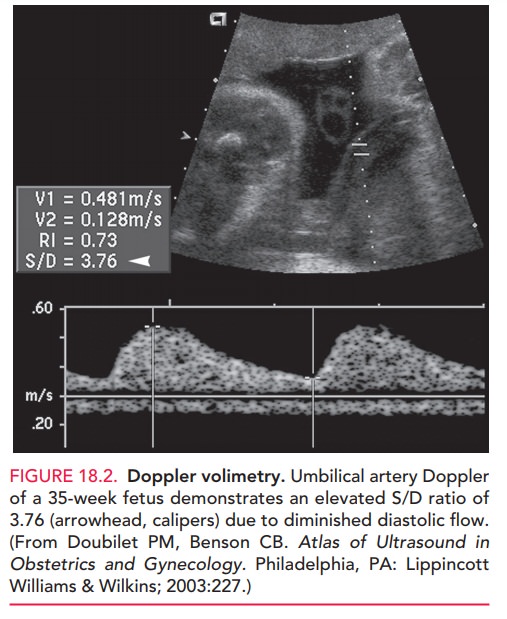
Doppler
velocimetry of fetal vessels provides furtherinsight into the
fetal response to altered growth, and has become part of the standard
assessment of the fetus once IUGR is diagnosed. Doppler velocimetry has been
shown to both reduce interventions and improve fetal outcome in pregnancies at
risk for IUGR. Fetal-placental circula-tion is evaluated in the umbilical
artery and is measured by a systolic/diastolic (S/D) ratio. The S/D indirectly
mea-sures impedance or resistance downstream within the pla-cental vessels. As
placental resistance increases, diastolic flow decreases and the S/D ratio
rises. A normal S/D ratioat term is 1.8
to 2.0. Fetuses with IUGR with absent or
The fetal middle cerebral
artery is also evaluated and reflects fetal adaptation. The patho-physiologic
response to reduced placental perfusion gen-erally spares the fetal brain,
resulting in an increase of diastolic and mean blood flow velocity in the
middle cere-bral artery. Ductus venosus may also be evaluated by Doppler
ultrasound, and the fetus with abnormal ductus flow is at very high risk of
adverse outcome.
Management
The goal
of management of a growth-restricted fetus is to deliver the healthiest
possible infant at the optimal time. Continued management of pregnancy with
IUGR is based on the results of fetal testing.
Serial evaluations of fetal
biometry should be performed every 3 or 4 weeks to follow the extent of growth
restriction. Fetal monitoring is
important, and may include fetal move-ment counting, nonstress testing,
biophysical profiles, and Doppler studies. There are no specific therapies that
have proven beneficial for pregnancies complicated by IUGR.
The fetus
should be delivered if the risk of fetal death exceeds that of neonatal death,
although in many cases these risks are difficult to assess.
For example, a fetus with IUGR
with normal anatomic sur-vey, normal amniotic fluid volume, normal Doppler
studies, and normal fetal testing may not benefit from early delivery.
Conversely, the growth-restricted fetus with serial biometry measurements
documenting decreasing growth rate and/or mildly abnormal Doppler studies may
benefit from deliv-ery, with or without fetal maturity documentation.
Neonatal management of IUGR
infants may partially depend on gestational age, but includes preparation for
neonatal respiratory compromise, hypoglycemia, hypo-thermia, and hyperviscosity
syndrome. Growth-restricted fetuses have less fat deposition in late pregnancy,
so newborn euglycemia cannot be maintained by the normal mechanism of
mobilization of glucose by fat metabolism. Hyper-viscosity
syndrome results from the fetus’s attempt tocompensate for poor placental
oxygen transfer by increas-ing the hematocrit to more than 65%. After birth,
this marked polycythemia can cause multiorgan thrombosis, heart failure, and
hyperbilirubinemia. Overall, growth-restricted infants who survive the neonatal
period have a generally good prognosis.
Related Topics