Chapter: Modern Pharmacology with Clinical Applications: Insulin and Oral Drugs for Diabetes Mellitus
Insulin
INSULIN
More than a century has
passed since von Mering and Minkowski first demonstrated that pancreatectomized
dogs exhibited signs and symptoms characteristic of dia-betes mellitus. Shortly
thereafter, Banting and Best used pancreatic extracts to reverse these symptoms
in diabetic patients, thus providing a basis for establishing a
cause-and-effect relationship between insulin deficiency and diabetes. Insulin
was subsequently isolated, crystal-lized, and eventually synthesized in the
laboratory. Insulin replacement therapy has been widely used in the clinical
management of diabetes mellitus for more than 70 years. In 1982, recombinant
DNA (rDNA) derived human insulin was
first produced and is now widely used instead
of insulin derived from beef or pork. More re-cently, insulin analogues have
been produced that mod-ulate the activity and rate of insulin action.
Chemistry
Insulin is a relatively
simple protein consisting of 51 amino acids arranged as two polypeptide chains,
an α- chain and β-chain, connected by
disulfide bonds; the lat-ter are necessary to maintain tertiary structure and
bio-logical activity (Fig 67.1). Although the amino acid sequence and
composition of animal insulins may differ slightly from those of human insulin,
their biological ac-tions are similar. Alteration of specific amino acid
residues within the insulin molecule yields novel deriv-atives that vary in
their pharmacokinetics and binding affinity for the insulin receptor. Some
insulin analogues display mitogenic properties in addition to their meta-bolic
effects.
Biosynthesis and Secretion
The insulin molecule is
initially translated in pancreatic β-cells as a large single-chain polypeptide
called pre-proinsulin, then further
processed to proinsulin by spe-cific
endopeptidases and packaged into storage gran-ules prior to release. Proinsulin
has little inherent biological activity and must be converted to insulin by the
action of specific proteases in the Golgi apparatus; this enzyme action results
in the formation of insulin and C (connecting) peptide. C-peptide facilitates the correct folding of the α- and β-chains of insulin and
maintains the alignment of the disulfide bridges in in-sulin before cleavage of
the C-peptide from insulin. Both insulin and C-peptide are stored in the
pancreatic β-cell granules, and both are liberated during insulin se-cretion.
Though it is unclear whether C-peptide has any function after it enters the
circulation, it is sometimes measured as an indicator of endogenous insulin
pro-duction.
The specific stimulus for
insulin release involves fluctuations in the serum glucose levels and to a much
lesser extent levels of other substrates. Glucose enters the pancreatic β-cell
via glucose transporter isoform (GLUT) 4 glucose transporters, is quickly
phosphory-lated to glucose-6-phosphate, and triggers an intracellu-lar influx
of calcium ions that promotes fusion of the insulin-containing secretory
granules with the cell membrane (exocytosis).
Insulin is continuously secreted at a low basal level during
fasting, but a postprandial rise in serum glucose or amino acid levels can augment blood levels of insulin
severalfold. Other nutrients (e.g., arginine, leucine) and several hormones
(e.g., glucagon, growth hormone, secretin, gastrin cholecystokinin,
pancre-ozymin, adrenocorticotropin) modulate insulin release. The autonomic nervous
system also participates in the regulation of the rate of insulin secretion,
with the islets of Langerhans receiving both cholinergic and adrenergic
innervation. Insulin secretion is enhanced by vagal (cholinergic) and
diminished by sympathetic (adrenergic) stimulation.
Glucose-induced stimulation
of insulin release from cells is biphasic. The initial rapid rise in insulin
that fol-lows a rise in glucose is termed the first phase of insulin release
and is thought to reflect the release of the presynthesized insulin in the
storage granules; a more delayed and prolonged rise in insulin secretion
follows. This second phase of insulin secretion is due to an up-regulation of
insulin expression and production. The first phase of insulin secretion is
often blunted in diabetes.
Biochemical and Pharmacological Actions of Insulin
The biochemical actions of
insulin are complex and in-volve many steps to integrate carbohydrate, protein,
and lipid metabolism for the maintenance of fuel homeostasis. In addition to
its effects on stimulating glucose uptake by tissues, insulin has five major
physio-logical effects on fuel homeostasis. It can (1) diminish hepatic
glycogenolysis by inhibiting glycogen phospho-rylase; (2) promote hepatic
glucose storage into glyco-gen by stimulating glycogen synthetase; (3) inhibit
he-patic gluconeogenesis (i.e., convert noncarbohydrate substrates like amino
acids into glucose); (4) inhibit lipolysis by inhibiting hormone-sensitive
lipase activity, thereby decreasing plasma free fatty acid and glycerol levels;
and (5) promote the active transport of amino acids into cells for
incorporation into protein, thereby producing a net positive nitrogen balance.
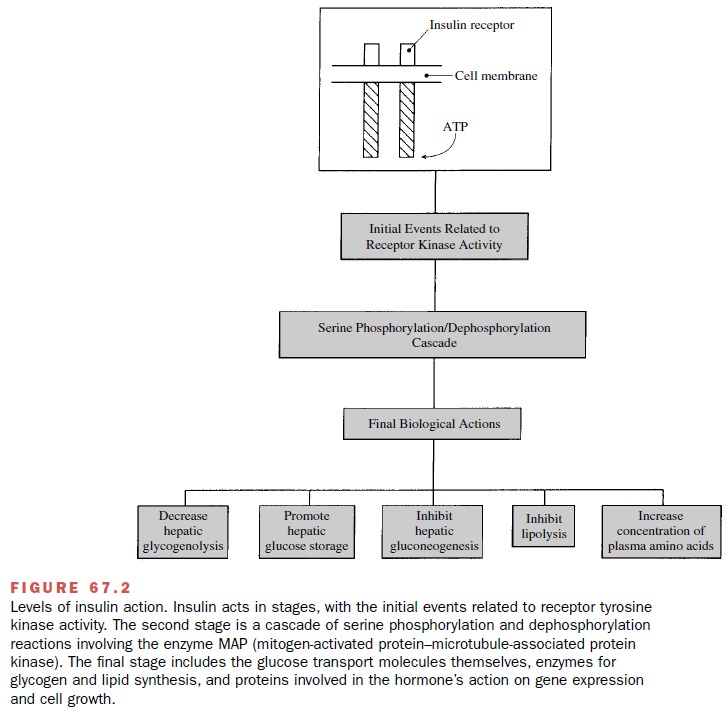
The biological actions of
insulin are initiated follow-ing a reversible binding of the hormone to a
high-affinity specific insulin receptor on the cell membrane surface (Fig.
67.2). The insulin receptor is a hetero-tetrameric tyrosine kinase receptor
composed of two α- and two β-subunits. Insulin binds to the α-subunit on the extracellular surface of the
cell and activates tyrosine ki-nase activity in the intracellular portion of
the β-subunit. This results in the
autophosphorylation of the adjacent insulin β-receptor subunit and the
phosphorylation of tyrosine residues on cytoplasmic proteins, termed the
in-sulin receptor substrate (IRS) 1 and 2. IRS phosphory-lation provides a
docking site for other intracellular sig-naling proteins. The regulatory
subunit of phosphatidyl inositol 3 (PI-3) kinase (p85) becomes activated and
dimerizes with its catalytic subunit (p110), and this com-plex mobilizes the
translocation of glucose transporters to the cell membrane surface, which
promotes hexose transport. Other downstream signaling pathways include
activation of p70-S6 kinase, protein kinase B (both via PI-3 kinase), and Grb2
activation of the Ras-Raf-MAP kinase pathway, which controls glycogen synthesis
and cell growth. The hormone–receptor complex may then be internalized by
endocytosis, which results in degrada-tion of insulin and recycling of the
receptor to the cell membrane surface.
Absorption, Metabolism, and Excretion
Insulin is usually administered subcutaneously. De-pending on the type of insulin being administered, the rate of insulin absorption can be modulated by altering the polymerization of the insulin molecule (e.g., monomers, dimers, or hexamers).
Intramuscular
injec-tions of insulin are used less often because absorption is more rapid.
Being a polypeptide hormone, insulin is readily inactivated if administered
orally. In emergen-cies, such as severe diabetic ketoacidosis, insulin can be
given intravenously. Clinical studies are examining the efficacy and safety of
inhaled insulin, which may be promising for some patients.
Once insulin enters the
circulation, its plasma half-life is less than 10 minutes. Hepatic insulinases
destroy approximately 50% of circulating insulin, with the re-mainder degraded
by circulating proteases. Therefore, only a relatively small amount of the
total endogenous insulin secreted ever reaches the peripheral tissues. Although
a number of tissues accumulate small amounts of insulin, the liver and kidney are the principal sites of hormone uptake and degradation. Insulin
metabolism is accomplished both
through the actions of an insulin-specific protease found in the cytosol of
many tissues and by the reductive cleavage of the insulin disulfide bonds by
glutathione–insulin transhydrogenase. In the kidney, insulin that undergoes
glomerular filtration is al-most completely reabsorbed and metabolized within
the proximal convoluted tubules of the nephron.
Related Topics