Chapter: Modern Pharmacology with Clinical Applications: Insulin and Oral Drugs for Diabetes Mellitus
Clinical Management of Diabetes
CLINICAL
MANAGEMENT OF DIABETES
Diet is the cornerstone of
the management of diabetes, regardless of the severity of the symptoms or the
type of diabetes. Exercise is also an important component in managing diabetes,
particularly in obese individuals with NIDDM who may have a component of
insulin re-sistance as a consequence of obesity. Treatment regi-mens that have
proved effective include a calorie-restricted diet in combination with
exogenous insulin or oral hypoglycemic drugs. However, since diet, exercise,
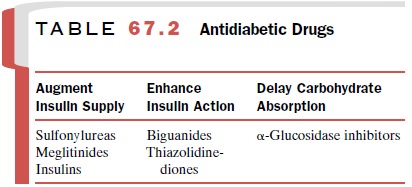
and oral hypoglycemic drugs (Table 67.2), often be-cause of noncompliance by the
patient, will not always achieve the clinical objectives of controlling the
symp-toms of diabetes, insulin remains universally important in therapeutic
management. The administration of in-sulin is required for the treatment of
type I (IDDM) and in cases of type II (NIDDM) that are refractory to management
with oral hypoglycemic drugs.
Because the spectrum of
patients with diabetes ex-tends from the totally asymptomatic individual to one
with life-threatening ketoacidosis, therapeutic
manage-ment must be highly individualized. An important ob-jective is to
maintain a glucose level as close to normal as possible without producing
frequent hypoglycemia or overly restricting the patient’s lifestyle. Many
diabet-ics aim to achieve an average blood glucose below 150 (hemoglobin A1c <
7%). Unstable or ketoacidosis-prone diabetics are difficult to maintain with a
single dose of either intermediate- or long-acting insulin; they usually
require multiple injections of combinations of short-, intermediate-, and/or long-acting
insulin prepa-rations.
Insulin Preparations
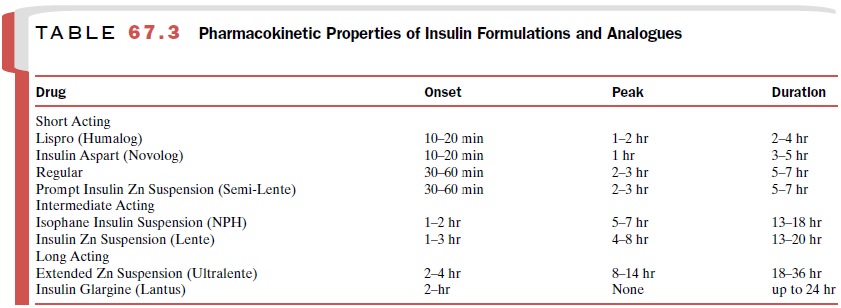
Commercially available
insulins differ in their onset of action, maximal activity, and duration of
action (Table 67.3). They can be classified as rapid acting (0–5 hours), short
acting (0–8 hours), intermediate
acting (2 to 16 hours), and long acting (4 to 36 hours). Human
insulin (e.g. Humulin, Novolin)
produced by rDNA technology is now widely available and has largely supplanted
in
Some insulins have been modified through genetic engineering to produce
insulin analogues, derivatives that possess novel phar-macokinetic properties
(lispro, insulin aspart, and in-sulin glargine). The duration of action can
vary with fac-tors such as injection volume, injection site, and blood flow at
the site of administration.
Rapid-acting insulin analogues (lispro, insulin aspart [Humalog, Novolog]) have
been engineered to contain amino acid modifications that promote rapid entry
into the circulation from subcutaneous tissue. They begin to exert their
effects as early as 5 to 10 minutes after admin-istration. Lispro insulin, the
first insulin analogue to be ap-proved in Europe and the United States, is
produced by switching the positions of lysine-proline amino acid residues 28
and 29 of the carboxy terminus of the β-chain. Lispro insulin displays very
similar actions to insulin and has a similar affinity for the insulin receptor,
but it cannot form stable hexamers or dimers in subcutaneous tissue, which
promotes its rapid uptake and absorption.
Insulin aspart is absorbed
nearly twice as fast as regular insulin. In addition to binding to the insulin
re-ceptor, insulin aspart also binds to the insulinlike growth factor (IGF-1)
receptor, which shares structural homology with the insulin receptor. However,
at physi-ological and pharmacological levels, the metabolic ef-fects of insulin
aspart predominate. Both lispro insulin and insulin aspart have relatively fast
onsets and short half-lives, making them ideal for controlling the upward
glycemic excursions that occur immediately after meals in diabetics.
Short-acting or regular insulins (Humulin R, Novolin R) take 30 minutes to
begin to exert their effect but have
a longer duration of action than does either lispro insulin or insulin aspart.
Typically, regular insulin is ad-ministered several minutes before a meal; it
has a more gradual onset of action and is designed to control post-prandial
hyperglycemia. Regular insulin is primarily used to supplement intermediate- and
long-acting in-sulin preparations; however, it is also the preparation of
choice for glucose management during surgery, trauma, shock, or diabetic
ketoacidosis. Regular insulin can be given intravenously when emergency
diabetes manage-ment is required (e.g., diabetes ketoacidosis). Prompt insulin
zinc suspension (Semilente) is also a
fast-acting form of insulin, but unlike regular insulin, it should be mixed
only with Lente or Ultralente insulin prepara-tions.
Rapid-acting and short-acting insulins are often administered two to three
times a day or more. These insulins are also employed in sliding scale insulin
regi-mens, which supplement a person’s glucose control based on blood glucose
monitoring equipment.
Intermediate-acting preparations (e.g., isophane in-sulin
suspension [NPH insulin] or insulin
zinc suspen-sion [Lente insulin])
have a more delayed onset of ac-tion, but they act longer. Conjugation of the
insulin molecule with either zinc or protamine or both will con-vert the
normally rapidly absorbed parenterally admin-istered insulin to a preparation
with a longer duration of action. Isophane insulin suspension (Neutral protamine Hagedorn, NPH) has a rate of absorption that has been slowed by complexing insulin with
protamine, a polyva-lent cation. Both NPH and Lente insulin are used to con-trol diabetes in a variety of
situations except during emergencies (e.g., diabetic ketoacidosis).
Intermediate-acting insulin preparations are usually given once or twice a day.
Protamine zinc and extended
insulin zinc suspension (Ultralente)
are often referred to as long-acting
insulin preparations. These insulins have more protamine and zinc in the
mixture than is found in isophane insulin sus-pension. Insulin zinc suspension,
extended (Ultralente Insulin), is quite similar to the
protamine zinc insulin suspension
except that it does not contain protamine. Both of these long-acting insulins
have an approximate duration of action of 36 hours.
Insulin glargine (Lantus) is a long-acting insulin
analogue that does not use zinc or protamine to modu-late insulin solubility.
The introduction of two positive arginine residues at the carboxy terminus of
the β-chain shifts the isoelectric point of the peptide from 5.4 to 6.7, thus
creating a molecule that is soluble at pH 4 but less soluble at neutral
(physiological) pH (in subcutaneous tissue). A second modification of insulin,
glargine, in-volves the substitution of a charge-neutral glycine for a
negatively charged asparagine at the amino terminal end of the β-chain; this
prevents deamidation and dimerization and enhances stability at physiological
pH. Injection of insulin glargine forms microprecipitates in subcutaneous
tissue as the pH is raised from 4 to phys-iological. A steady, sustained
release of insulin from the site of injection mimics the basal secretion of
insulin from the pancreas. Absorption of insulin glargine com-mences within a
few hours of injection, and there is usu-ally little or no peak or trough in
the levels of insulin glargine as it dissolves from its site of injection.
Because it is necessary to maintain its acidic pH prior to injec-tion, insulin
glargine must not be mixed with any other form of insulin during injection.
Adverse Reactions to Insulin Therapy
The most common side effect
associated with insulin therapy is hypoglycemia,
which may result in such CNS symptoms as tremors, lethargy, hunger, confusion,
mo-tor and sensory deficits, seizures, and unconsciousness. Adrenergic
manifestations include anxiety, palpitations, tachycardia, and diaphoresis. In
many cases, diabetics are aware that hypoglycemia is developing, and prompt
administration of oral carbohydrates (e.g., fruit juice or glucose tablets) can
restore normoglycemia. In more se-vere cases (e.g., unconsciousness, seizures),
intravenous glucose or intramuscular glucagon is required to reverse the
hypoglycemia.
Another frequent side effect
of insulin therapy is weight gain. Some is due to increased caloric storage of
glucose by insulin, and some is due to renal sodium re-tention resulting in
fluid retention and edema. These ef-fects can synergize with oral agents that
are often coad-ministered with insulin, particularly sulfonylureas and
thiazolidinediones.
Other complications arising
from insulin therapy are uncommon. Sometimes, diabetics treated with exogenous
insulin develop insulin-binding immunoglobulins, although the clinical
significance of these antibodies remains unclear. Allergic reactions due to the
use of animal-derived insulins has subsided since the use of re-combinant
DNA-derived human insulin became wide-spread. Over time, repeated subcutaneous
injections of insulin can cause local lipodystrophy (lipohypertrophy or
lipoatrophy), which may alter the pharmacokinetics of insulin absorption from
this site. Also, hypokalemia can follow acute insulin administration, an effect
that is due to the stimulation of NA+ –K+ –ATPase
(adenosine triphosphatase) with its resultant redistribution of K+ to
the intracellular compartment. This property of insulin is sometimes used in
the emergency treatment of hy-perkalemia.
Insulin Regimens
The rational design of
insulin regimens involves esti-mates and consideration of the patient’s diet,
lifestyle, level of physical activity, and type of diabetes. A thin, ac-tive
type I diabetic will have very different insulin re-quirements from those of a
sedentary, obese type II di-abetic. Hence, it is not possible to provide a
cookbook approach for designing all diabetes regimens. There is usually less
insulin resistance in type I diabetics, and it is possible to estimate
metabolic needs of insulin based on the type I diabetic patient’s weight
(typically 0.5 to 1 units/kg/day). Other considerations, such as work schedule
and mealtimes, are important in determining the way the insulin is divided
proportionally to cover short-range and long-range glycemic control. Although
there is quite a bit of variation, most diabetics have about half to two-thirds
of their insulin as a long-acting preparation, and the rest is usually
delivered as a rapid-or short-acting insulin.
Some insulin preparations are
combinations of NPH and regular
insulin packaged in premixed ratios of 70:30 or 50:50 of NPH and regular insulin (70/30 Humulin, 70/30 Novolin,
50/50 Humulin). A similar combination
product is 75/25 insulin, which contains 75% protamine lispro and 25%
lispro insulin. Insulin zinc suspension (Lente
insulin) is an intermediate-acting mixture of prompt insulin zinc suspension
(30%) and extended in-sulin zinc suspension (70%). While these combination products
may be convenient for some patients and can improve compliance, they are not
ideal regimens for most diabetics, who may achieve better control by
sep-arately mixing their rapid- or short-acting insulin with an intermediate-
or long-acting insulin to arrive at a ra-tio that is better suited to manage
their diabetes.
Insulin pumps are small, portable devices worn ex-ternally that deliver a
continuous supply of insulin sub-cutaneously through a hypodermic needle. The
pumps provide a basal rate of insulin between meals and can be manually
adjusted to facilitate glycemic control at mealtimes. Rapid and short-acting
insulins are typically used in insulin pumps. Pumps are usually worn 2 to 3
days before the tubing and needle are changed.
Related Topics