Chapter: Basic & Clinical Pharmacology : Management of the Poisoned Patient
Initial Management of the Poisoned Patient
INITIAL MANAGEMENT OF THE POISONED PATIENT
The initial management
of a patient with coma, seizures, or oth-erwise altered mental status should
follow the same approach regardless of the poison involved: supportive measures
are the basics (“ABCDs”) of
poisoning treatment.
First,
the airway should be cleared of vomitus or any other obstruction and an oral
airway or endotracheal tube inserted if needed. For many patients, simple
positioning in the lateral, left-side-down position is sufficient to move the
flaccid tongue out of the airway. Breathing should be assessed by observation
and pulse oxi-metry and, if in doubt, by measuring arterial blood gases.
Patients with respiratory insufficiency should be intubated and mechanically
ventilated. The circulation should be assessed by continuous moni-toring of
pulse rate, blood pressure, urinary output, and evaluation of peripheral
perfusion. An intravenous line should be placed and blood drawn for serum
glucose and other routine determinations.
At this point, every
patient with altered mental status should receive a challenge with concentrated
dextrose, unless a rapid bedside
blood glucose test demonstrates that the patient is not hypoglycemic. Adults
are given 25 g (50 mL of 50% dextrose solution) intravenously, children 0.5
g/kg (2 mL/kg of 25% dex-trose). Hypoglycemic patients may appear to be
intoxicated, and there is no rapid and reliable way to distinguish them from
poi-soned patients. Alcoholic or malnourished patients should also receive 100
mg of thiamine intramuscularly or in the intravenous infusion solution at this
time to prevent Wernicke’s syndrome.
The
opioid antagonist naloxone may be
given in a dose of 0.4–2 mg intravenously. Naloxone reverses respiratory and
CNS depression due to all varieties of opioid drugs . It is useful to remember
that these drugs cause death primarily by respiratory depression; therefore, if
airway and breathing assistance have already been instituted, naloxone may not
be necessary. Larger doses of naloxone may be needed for patients with overdose
involv-ing propoxyphene, codeine, and some other opioids. The benzodi-azepine
antagonist flumazenil may be of value in patients with suspected
benzodiazepine overdose, but it should not be used if there is a history of
tricyclic antidepressant overdose or a seizure disorder, as it can induce
convulsions in such patients.
History & Physical Examination
Once the essential
initial ABCD interventions have been instituted, one can begin a more detailed
evaluation to make a specific diagno-sis. This includes gathering any available
history and performing a toxicologically oriented physical examination. Other
causes of coma or seizures such as head trauma, meningitis, or metabolic
abnor-malities should be sought and treated. Some common intoxications are
described under Common Toxic Syndromes.
A. History
Oral statements about
the amount and even the type of drug ingested in toxic emergencies may be
unreliable. Even so, family members, police, and fire department or paramedical
personnel should be asked to describe the environment in which the toxic
emergency occurred and should bring to the emergency depart-ment any syringes,
empty bottles, household products, or over-the-counter medications in the
immediate vicinity of the possibly poisoned patient.
B. Physical Examination
A brief examination
should be performed, emphasizing those areas most likely to give clues to the
toxicologic diagnosis. These include vital signs, eyes and mouth, skin,
abdomen, and nervous system.
· Vital signs—Careful evaluation of vital
signs (blood pressure,pulse, respirations, and temperature) is essential in all
toxicologic emergencies. Hypertension and tachycardia are typical with
amphetamines, cocaine, and antimuscarinic (anticholinergic)
drugs. Hypotension and
bradycardia are characteristic features of overdose with calcium channel
blockers, β
blockers, clonidine, and sedative hypnotics. Hypotension with tachycardia is
commonwith tricyclic antidepressants, trazodone, quetiapine, vasodilators, and β agonists. Rapid
respirations are typical of salicylates, carbon monoxide, and other toxins that
produce metabolic acidosis or cellular asphyxia. Hyperthermia may be associated
with sympath-omimetics, anticholinergics, salicylates, and drugs producing
sei-zures or muscular rigidity. Hypothermia can be caused by any CNS-depressant
drug, especially when accompanied by exposure to a cold environment.
· Eyes—The eyes
are a valuable source of toxicologic information.Constriction of the pupils
(miosis) is typical of opioids, clonidine, phenothiazines, and cholinesterase
inhibitors (eg, organophosphate insecticides), and deep coma due to sedative
drugs. Dilation of the pupils (mydriasis) is common with amphetamines, cocaine,
LSD, and atropine and other anticholinergic drugs. Horizontal nystagmus is
characteristic of intoxication with phenytoin, alcohol, barbiturates, and other
sedative drugs. The presence of both vertical and horizon-tal nystagmus is
strongly suggestive of phencyclidine poisoning. Ptosis and ophthalmoplegia are
characteristic features of botulism.
· Mouth—The mouth may show
signs of burns due to corro-sive substances, or soot from smoke inhalation.
Typical odors of alcohol, hydrocarbon solvents, or ammonia may be noted.
Poisoning due to cyanide can be recognized by some examiners as an odor like
bitter almonds.
· Skin—The skin often appears
flushed, hot, and dry in poison-ing with atropine and other antimuscarinics.
Excessive sweating occurs with organophosphates, nicotine, and sympathomimetic
drugs. Cyanosis may be caused by hypoxemia or by methemoglo-binemia. Icterus
may suggest hepatic necrosis due to acetamino-phen or Amanita phalloides mushroom poisoning.
· Abdomen— Abdominal examination
may reveal ileus, whichis typical of poisoning with antimuscarinic, opioid, and
sedative drugs. Hyperactive bowel sounds, abdominal cramping, and diar-rhea are
common in poisoning with organophosphates, iron, arsenic, theophylline, A phalloides, and A muscaria.
· Nervous system—A careful
neurologic examination is essen-tial. Focal seizures or motor deficits suggest
a structural lesion (eg, intracranial hemorrhage due to trauma) rather than
toxic or meta-bolic encephalopathy. Nystagmus, dysarthria, and ataxia are
typi-cal of phenytoin, carbamazepine, alcohol, and other sedative intoxication.
Twitching and muscular hyperactivity are common with atropine and other
anticholinergic agents, and cocaine and other sympathomimetic drugs. Muscular
rigidity can be caused by haloperidol and other antipsychotic agents, and by
strychnine or by tetanus. Generalized hypertonicity of muscles and lower
extremity clonus are typical of serotonin syndrome. Seizures areoften caused by
overdose with antidepressants (especially tricyclic antidepressants and
bupropion [as in the case study]), cocaine, amphetamines, theophylline,
isoniazid, and diphenhydramine. Flaccid coma with absent reflexes and even an
isoelectric electro-encephalogram may be seen with deep coma due to
sedative-hypnotic or other CNS depressant intoxication and may be mistaken for
brain death.
Laboratory & Imaging Procedures
A. Arterial Blood Gases
Hypoventilation
results in an elevated PCO2
(hypercapnia) and a low PO2 (hypoxia).
The PO2
may also be low in a patient with aspiration pneumonia or drug-induced
pulmonary edema. Poor tissue oxygenation due to hypoxia, hypotension, or
cyanide poisoning will result in metabolic acidosis. The PO2
measures only oxygen dissolved in the plasma and not total blood oxygen content
or oxyhemoglobin saturation and may appear normal in patients with severe
carbon monoxide poisoning. Pulse oxi-metry may also give falsely normal results
in carbon monoxide intoxication.
B. Electrolytes
Sodium, potassium,
chloride, and bicarbonate should be mea-sured. The anion gap is then calculated
by subtracting the mea-sured anions from cations:
Anion gap = (Na+ + K+) – (HCO3– + Cl–)
Normally,
the sum of the cations exceeds the sum of the anions by no more than 12–16
mEq/L (or 8–12 mEq/L if the formula used for estimating the anion gap omits the
potassium level). A larger-than expected anion gap is caused by the presence of
unmeasured anions (lactate, etc) accompanying metabolic acidosis. This may
occur with numerous conditions, such as diabetic ketoacidosis, renal failure,
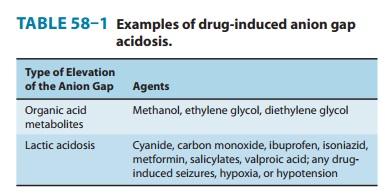
or shock-induced lactic acidosis. Drugs that may induce an elevated anion gap
metabolic acidosis (Table 58–1) include aspirin, metformin, methanol, ethylene
glycol, isoniazid, and iron.
Alterations in the serum potassium level are hazardous because they can result in cardiac arrhythmias. Drugs that may cause hyper-kalemia despite normal renal function include potassium itself, β blockers, digitalis glycosides, potassium-sparing diuretics, and fluo-ride. Drugs associated with hypokalemia include barium, β ago-nists, caffeine, theophylline, and thiazide and loop diuretics.
C. Renal Function Tests
Some toxins have
direct nephrotoxic effects; in other cases, renal failure is due to shock or
myoglobinuria. Blood urea nitrogen and creatinine levels should be measured and
urinalysis performed. Elevated serum creatine kinase (CK) and myoglobin in the
urine suggest muscle necrosis due to seizures or muscular rigidity. Oxalate
crystals in large numbers in the urine suggest ethylene glycol poisoning.
D. Serum Osmolality
The calculated serum
osmolality is dependent mainly on the serum sodium and glucose and the blood
urea nitrogen and can be estimated from the following formula:
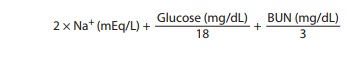
This calculated value
is normally 280–290 mOsm/L. Ethanol and other alcohols may contribute
significantly to the measured serum osmolality but, since they are not included
in the calcula-tion, cause an osmol gap:
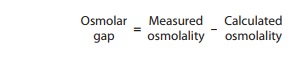
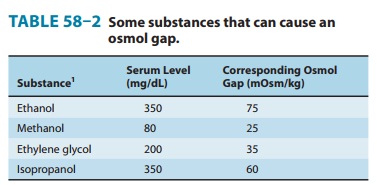
Table 58–2 lists the
concentration and expected contribution to the serum osmolality in ethanol,
methanol, ethylene glycol, and isopropanol poisonings.
E. Electrocardiogram
Widening of the QRS complex duration (to more than 100 milli-seconds) is typical of tricyclic antidepressant and quinidine over-doses (Figure 58–1). The QTc interval may be prolonged (to more than 440 milliseconds) in many poisonings, including quinidine, antidepressants and antipsychotics, lithium, and arsenic (see also http://www.torsades.org/). Variable atrioventricular (AV) block and a variety of atrial and ventricular arrhythmias are common with poisoning by digoxin and other cardiac glycosides. Hypoxemia due to carbon monoxide poisoning may result in ischemic changes on the electrocardiogram.
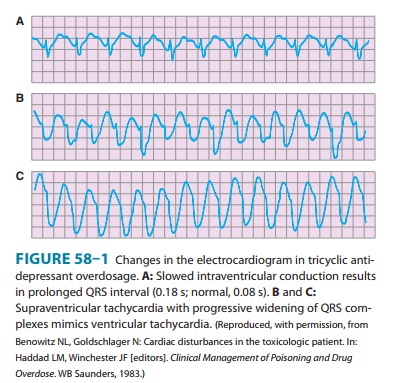
F. Imaging Findings
A plain film of the
abdomen may be useful because some tablets, particularly iron and potassium,
may be radiopaque. Chest radio-graphs may reveal aspiration pneumonia,
hydrocarbon pneumo-nia, or pulmonary edema. When head trauma is suspected, a
computed tomography (CT) scan is recommended.
Toxicology Screening Tests
It is a common
misconception that a broad toxicology “screen” is the best way to diagnose and
manage an acute poisoning. Unfortunately, comprehensive toxicology screening is
time-consuming and expensive and results of tests may not be available for
days. Moreover, many highly toxic drugs such as calcium chan-nel blockers, β blockers, and
isoniazid are not included in the screening process. The clinical examination
of the patient and selected routine laboratory tests are usually sufficient to
generate a tentative diagnosis and an appropriate treatment plan. Although
screening tests may be helpful in confirming a suspected intoxica-tion or for
ruling out intoxication as a cause of apparent brain death, they should not
delay needed treatment.
When a specific
antidote or other treatment is under consid-eration, quantitative laboratory
testing may be indicated. For example, determination of the acetaminophen level
is useful in assessing the need for antidotal therapy with acetylcysteine.
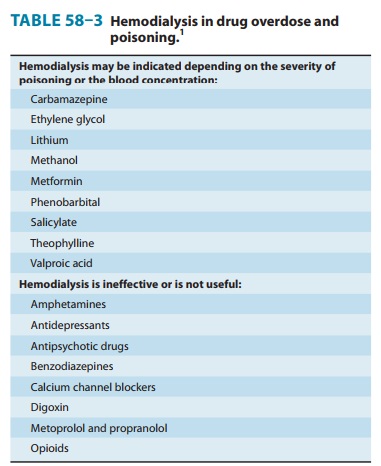
Serum levels of salicylate (aspirin), ethylene glycol, methanol, theophylline,
carbamazepine, lithium, valproic acid, and other drugs and poisons may indicate
the need for hemodialysis (Table 58–3).
Decontamination
Decontamination
procedures should be undertaken simultane-ously with initial stabilization,
diagnostic assessment, and labora-tory evaluation. Decontamination involves
removing toxins from the skin or gastrointestinal tract.
A. Skin
Contaminated clothing
should be completely removed and double-bagged to prevent illness in health
care providers and for possible laboratory analysis. Wash contaminated skin
with soap and water.
B. Gastrointestinal Tract
Controversy
remains regarding the efficacy of gut emptying by emesis or gastric lavage,
especially when treatment is initiated more than 1 hour after ingestion. For
most ingestions, clinical toxicologists recommend simple administration of
activated char-coal to bind ingested poisons in the gut before they can be
absorbed (as in the case study). In unusual circumstances, induced emesis or
gastric lavage may also be used.
·
Emesis—Emesis can
be induced with ipecacsyrup(neverextract of ipecac), and this method was
previously used to treatsome childhood ingestions at home under telephone
supervision of a physician or poison control center personnel. However, the
risks involved with inappropriate use outweighed the unproven benefits, and
this treatment is rarely used in the home or hospital. Ipecac should not be
used if the suspected intoxicant is a corrosive agent, a petroleum distillate,
or a rapid-acting convulsant. Previously popular methods of inducing emesis
such as fingertip stimulation of the pharynx, salt water, and apomorphine are
inef-fective or dangerous and should not be used.
·
Gastric lavage—If the patient is
awake or if the airway isprotected by an endotracheal tube, gastric lavage may
be per-formed using an orogastric or nasogastric tube—as large a tube as
possible. Lavage solutions (usually 0.9% saline) should be at body temperature
to prevent hypothermia.
·
Activated charcoal—Owing to its large
surface area, acti-vated charcoal can adsorb many drugs and poisons. It is most
effective if given in a ratio of at least 10:1 of charcoal to estimated dose of
toxin by weight. Charcoal does not bind iron, lithium, or potassium, and it
binds alcohols and cyanide only poorly. It does not appear to be useful in
poisoning due to corrosive mineral acids and alkali. Studies suggest that oral
activated charcoal given alone may be just as effective as gut emptying (eg,
ipecac-induced emesis or gastric lavage) followed by charcoal. Repeated doses of
oral activated charcoal may enhance systemic elimination of some drugs
(including carbamazepine, dapsone, and theophylline) by a mechanism referred to
as “gut dialysis,” although the clinical ben-efit is unproved.
· Cathartics— Administration
of a cathartic (laxative) agentmay hasten removal of toxins from the
gastrointestinal tract and reduce absorption, although no controlled studies
have been done. Whole bowel irrigation with a balanced polyethylene
glycol-electrolyte solution (GoLYTELY, CoLyte) can enhance gut decon-tamination
after ingestion of iron tablets, enteric-coated medicines, illicit drug-filled
packets, and foreign bodies. The solution is administered orally at 1–2 L/h
(500 mL/h in children) for several hours until the rectal effluent is clear.
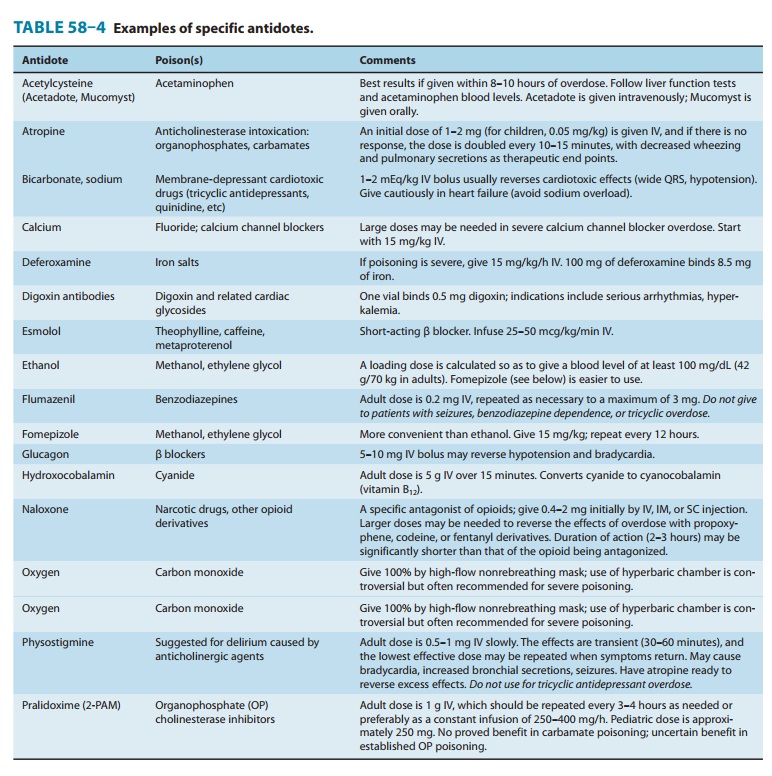
Specific Antidotes
There is a popular
misconception that there is an antidote for every poison. Actually, selective
antidotes are available for only a few classes of toxins. The major antidotes
and their characteristics are listed in Table 58–4.
Methods of Enhancing Elimination of Toxins
After appropriate
diagnostic and decontamination procedures and administration of antidotes, it
is important to consider whether measures for enhancing elimination, such as
hemodialysis or uri-nary alkalinization, can improve the clinical outcome.
Table 58–3 lists intoxications for which dialysis may be beneficial.
A. Dialysis Procedures
·
Peritoneal dialysis—Although it is a
relatively simple andavailable technique, peritoneal dialysis is inefficient in
removing most drugs.
·
Hemodialysis—Hemodialysis is more
efficient than perito-neal dialysis and has been well studied. It assists in
correction of fluid and electrolyte imbalance and may also enhance removal of
toxic metabolites (eg, formic acid in methanol poisoning; oxalic and glycolic
acids in ethylene glycol poisoning). The efficiency of both peritoneal dialysis
and hemodialysis is a function of the molecular weight, water solubility,
protein binding, endogenous clearance, and distribution in the body of the
specific toxin. Hemodialysis is especially useful in overdose cases in which
the precipitating drug can be removed and fluid and electrolyte imbal-ances are
present and can be corrected (eg, salicylate intoxication).
B. Forced Diuresis and Urinary pH Manipulation
Previously
popular but of unproved value, forced diuresis may cause volume overload and
electrolyte abnormalities and is not recom-mended. Renal elimination of a few
toxins can be enhanced by alteration of urinary pH. For example, urinary
alkalinization is use-ful in cases of salicylate overdose. Acidification may
increase the urine concentration of drugs such as phencyclidine and
amphet-amines but is not advised because it may worsen renal complications from
rhabdomyolysis, which often accompanies the intoxication.
Related Topics