Chapter: 11th Biochemistry : Chapter 4 : Enzymes
Inhibitors and its types
Inhibitors
An
inhibitor is defined as a substance which binds with the enzyme and brings
about a decrease in catalytic activity of that enzyme. For example,
anti-oxidants are added as inhibitors to food to retard its spoilage on
exposure to air (oxygen) and inhibition could be either reversible or
irreversible.
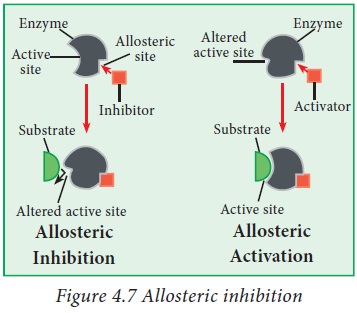
Allosteric activators and inhibitors:
This
type of inhibition takes place due to the presence of allosteric site (Greek
allo
‘other’;
stereos = ‘space’ or ‘site’) on the surface of the enzyme away from the active
site. The final end-product fits in the allosteric site and in some way brings
about a change in shape of the enzyme so that the active site of the enzyme
becomes unfit for making a complex with its substrate. Allosteric inhibition
may be reversible. In many metabolic reactions, when the concentration of the
final end product (usually acts as an allosteric inhibitor) in the cell falls
and the activity of the enzyme is restored. Similary an enzyme can also be
activated by an activator that binds to an allosteric site. This activator is
called as an allosteric activator.
Types of Inhibition
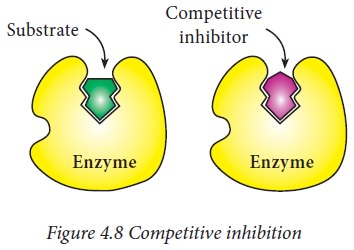
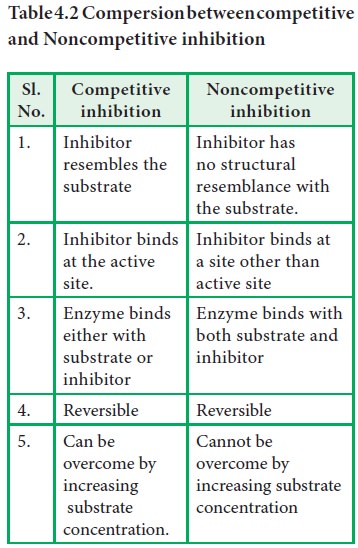
i) Competitive Inhibition
A
competitive inhibition usually is reversible. A competitive inhibitor usually
closely resembles the substrate and is regarded as substrate analogue. The
inhibitor competes with substrate and binds at the active site of the enzyme
but does not undergo any catalysis. As long as the competitive inhibitor is
bound to the active site, the enzyme will not be available for the substrate to
bind. This type of inhibition can be reversed by increasing the concentration
of substrate.
Example: 1) Enzyme - Xanthine oxidase; Substrate - Hypoxanthine; Inhibitor – Allopurinol.
Significance
of the inhibitor: Used in the control of Gout to reduce excess production of uric
acid from hypoxanthine.
Example: 2) Enzyme - Succinate dehydrogenase;
Substrate - Succnate; Inhibitor - Malonate
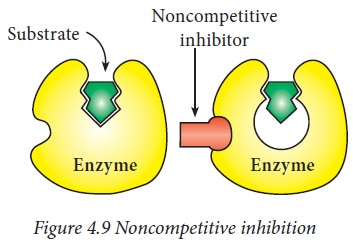
ii) Noncompetitive inhibition:
Usually
a noncompetitive inhibitor binds either to free enzyme or to ES complex at a
site other than the active site on the enzyme surface. This results in the
change of conformation of the enzyme as well as its active site, which makes
the substrate unable to bind to the enzyme effectively. This type of inhibitor
has no structural resemblance with the substrate like competitive inhibitors.
Non-competitive
inhibitors do not interfere with the enzyme-substrate binding. But catalysis is
prevented, possibly due to the distortion of enzyme conformation.
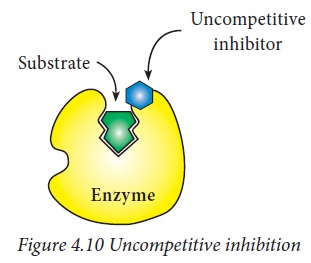
iii) Uncompetitive inhibition:
Uncompetitive
inhibitors binds only to the ES complex. However, the binding of the inhibitor
affects the binding of the substrate. This type of inhibition cannot be
overcome. The inhibitor usually follows an allosteric effect where it binds to
a different site on the enzyme than the substrate. This binding to an
allosteric site changes the conformation of the enzyme so that the affinity of
the substrate for the active site is reduced.
Related Topics