Chapter: Essential Clinical Immunology: Immunological Aspects of Renal Disease
Immunological Aspects of Renal Disease
Immunological Aspects of Renal
Disease
INTRODUCTION
Human kidneys play an integral role in the
development of primary or secondary immunologic diseases. As a major filter-ing
organ, the kidneys, which represent about 0.5 percent of the human body mass,
receive 20 percent of the total cardiac out-put. The enormous blood flow (1
L/min) to the renal microcirculation exceeds that observed in other major
vascular organs (heart, liver, and brain). Urine is produced after a complex
process of glomerular fil-tration, tubular transport, and reabsorption at a
rate of 1 ml/min. Cellular elements involved in immunity thereby have a high
probability of interacting with glomeru-lar and tubular cells that may or may
not cause renal disease.
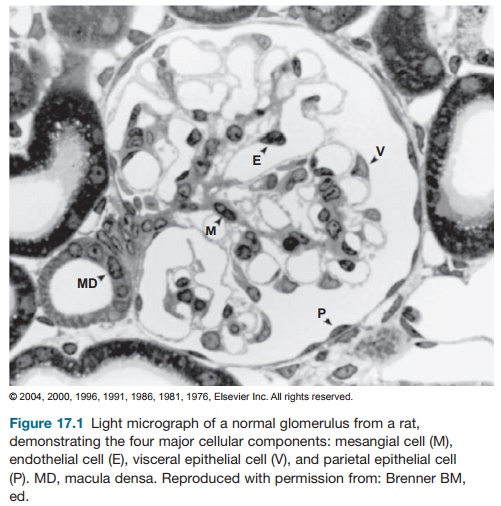
Sufficient knowledge of the anatomy and histology
of the kidney is vital in understanding the pathogenesis of renal diseases. The
renal corpuscle, or glomeru-lus (Figure 17.1), is composed of capillary tuft
lined by a thin layer of endothelial cells; central region of mesangial cells
and its surrounding matrix; and visceral and parietal epithelial cells with
their respec-tive basement membranes. The glomeru-lus is primarily responsible
for production of ultrafilrate from the circulating plasma. The filtration barrier
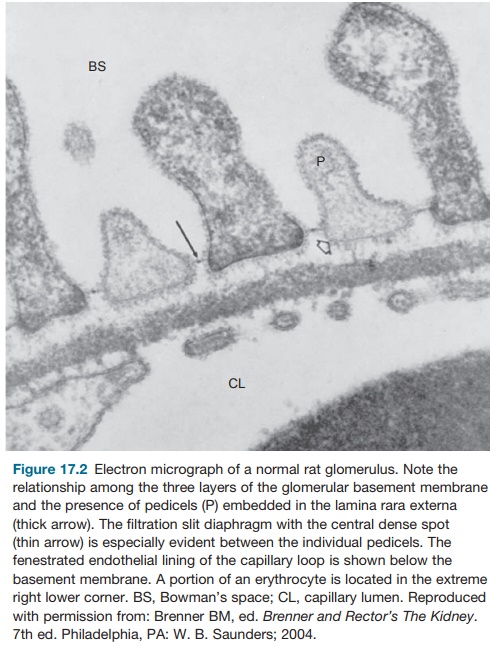
between the blood-stream and urinary space is made up by the fenestrated
endothelium, the glomeru-lar basement membrane (GBM), and the slit pores seen
between the foot processes of the visceral epithelium (Figure 17.2).
The endothelial cells form as initial barriers to
cellular elements of the blood (red blood cells, leucocytes, and platelets) in
reaching the subendothelial space. The endothelial cells produce nitric oxide
(a vasodilator) and endothelin-1 (a potent vasoconstrictor), chemical substances
implicated in inflammatory processes. The surface of the endothelial cells is
negatively charged, which may contribute to the charge-selective properties of
the glomeru-lar capillary wall.
The GBM is composed of a central dense layer called
the lamina densa and two thin layers
called the lamina rara externa and lamina rara interna. The GBM is formed
by the fusion of the endothelial and epithe-lial basement membrane during
develop-ment. Biochemical analyses of the GBM have identified the presence of
glycopro-teins (type IV collagen, laminin, fibronec-tin, and
endotactin/nidogen) and heparan sulfate proteoglycans (perlecan and agrin).
Type IV collagen is the major constituent of the GBM. Gene mutations involving
those encoding α3, α4, and α5 isomeric chains of the type IV
collagen can cause Alport’s syndrome. This is a progressive form of
glomerulopathy associated with ocular abnormalities, hearing loss, and
microscopic hematuria. Electron micro-scopic examination shows thinning of the
GBM in early stages of the disease. With
The presence of glycosaminoglycans rich in heparin
sulfate renders the GBM to have an anionic charge. Combined with the negatively
charged endothelial cell lining and the epithelial slit diaphragm, the GBM
becomes a formidable sieve that is both size and charge selective. Although it
restricts passage of large molecules like albumin, it allows small molecules
and large cationic molecules like ferritin to pass through. Enzymatic digestion
of the glycosaminoglycans increases permeability to large molecules like bovine
serum albumin. This strongly suggests that glycosaminoglycans play a
significant role in the permeability proper-ties of the GBM.
The visceral epithelial cells, or podo-cytes, wrap
around individual capillary loops to form pedicels, or foot processes, that
come in direct contact with the lam-ina rara externa of the GBM. The gaps
between the podocytes become the slit pore, which is bridged by a thin
mem-brane called filtration slit membrane,
or slit diaphragm (Figure 17.2). It appears that two membrane proteins, nephrin and CD2-associated protein (CD2AP), are
involved in maintaining the integrity of the filtra-tion slit membrane. The
CD2AP has been identified as an adapter molecule that binds nephrin to the
cytoskeleton of the GBM. Deletion of the CD2AP is known to cause congenital
nephrotic syndrome with morphologic evidence of effacement or fusion of foot
processes. Other mem-brane proteins like the human glomerular
Animal experiments that cause efface-ment or fusion of foot processes
(such as injection of antipodoplanin IgG antibod-ies in rats) or disruption of
the negatively charged GBM causes proteinuria. In addi-tion, visceral
epithelial cells are capable of endocytosis, can synthesize and maintain the
GBM, and produce prostaglandins.
Mesangial cells are specialized peri-cytes with
functional properties similar to that of smooth muscle cells. In addition to providing
structural support for the glo-merular capillary loop, its contractile
prop-erties help regulate glomerular filtration. The presence of actin and
myosin allows the mesangial cells to contract in the pres-ence of vasoactive
agents like angiotensin II, vasopressin, norepinephrine, leukotri-enes,
thromboxanes, and platelet-activat-ing factors. However, prostaglandins, atrial
peptides, and dopamine cause mesangial relaxation. The surrounding mesangial
matrix consists of glycosaminoglycans, fibronectins, laminin, and other
collagens. The presence of cell surface receptors in the matrix (e.g., β-integrin receptor) are impli-cated in signal
transduction mechanisms that promote synthesis of various inflam-matory
cytokines, vasoactive substances, and growth factors. Finally, mesangial cells
have phagocytic properties. During endocytosis of immune complexes,
intra-cellular production of prostaglandins and reactive oxygen species cause
injury to the glomerulus.
Parietal cells are squamous cells that make up the
epithelium that forms the outer wall of the Bowman’s capsule. At the vascular
pole, the parietal epithelium is continuous with the visceral epithelium. At
the urinary pole, there is a transition to the cuboidal cells of the proximal
tubule. The exact role of parietal cells is not well defined. However, as
pointed out by Brenner (2004), these cells can proliferate and become crescents
like in rapidly pro-gressive glomerulonephritis (RPGN).
Related Topics