Chapter: Essential Clinical Immunology: Immune-Mediated Neurological Syndromes
Animal Models of Central Nervous System
ANIMAL MODELS
A unique difficulty in developing treatment
strategies and pathogenic theories of neu-rological immune diseases is that
often the human end organ is unavailable for study. The morbidity of taking CNS
tissue purely for research places that option beyond ethical boundaries in most
cases. There-fore, reliance is mainly on autopsy mate-rial, when usually only
end-stage disease pathology is available, keeping the natural history of the
disease unknown. Therefore, reliance on animal models in these diseases
is paramount in testing treatment strate-gies and
theories of etiology. Controversy arises when one considers how closely the
animal model follows the human dis-ease state under study. The animal model
should reflect the susceptibility of the host, and the clinical findings of the
disease, and should be similar to the histopathological findings in the human.
MS and EAE provide good examples of strengths and
difficulties of animal models based on differences between human and murine
immunology. For example, in EAE IFN-ő≥ was
demonstrated in several studies to be a protective cytokine. Antibodies to the
cytokine exacerbated EAE, possibly by blocking induction and activation of
sup-pressor activity. However, when this prin-ciple was
applied to patients, the study was discontinued because of exacerbation of MS
by administration of IFN-ő≥ in a
clinical trial.
IFN-őĪ is another example of differ-ing
cytokine responses. In humans, this cytokine is secreted by several types of
cells, inclining macrophages in response to exposure to viral antigens. The
cyto-kine induces T cells to TH1 development, a process that depends
on signal transducer and transcription-4 (STAT-4) activation. In mice, however,
INF-őĪ dose not
activate STAT-4, therefore; TH1 is not induced by IFN-őĪ. Multiple other differences
abound in the immune systems between mice and humans; therefore, responses to
treatment protocols in models compared with human disease may vary greatly.
Differences
in the immunology of mice and human are numerous (see Table 16.2). For example,
in delayed-type hypersensi-tivity (DTH) in humans, neutrophils are the first
responders, followed by a mix of mononuclear cells composed of T cells and
macrophages. In mice, there are relatively
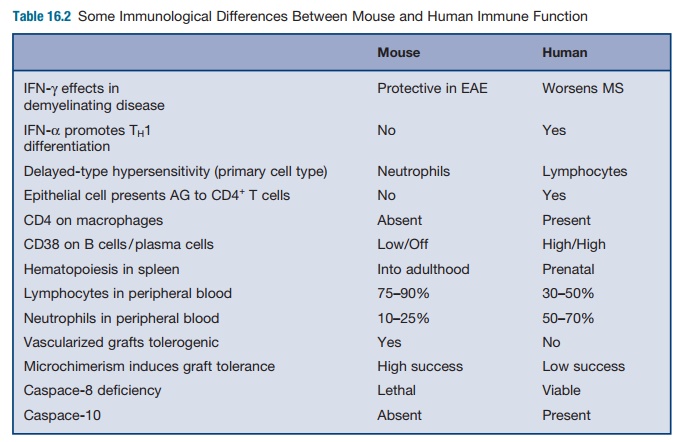
far fewer
neutrophils in the peripheral blood system than in humans. However, the murine
response to antigens in DTH is richer in neutrophils compared with humans and a
far greater amount of anti-gen is needed to elicit a response.
Human endothelial cells are now believed to be
antigen-presenting cells to memory CD4+ and CD8+ T
lymphocytes. This is not the case for CD8+ cells in mice. Therefore, in
humans, antigen transport to lymphoid tissue by Langerhans cells may not be
required for DTH, and the endothelial cells may locally trigger a response to
antigen. The peripheral lym-phoid system would be required in mice for DTH,
compared with local presentation in humans.
Human and mouse endothelial cells both express MHC
class I molecules, but only humans express class II, as well as CD40, ICOS
ligand 50, and CD58. This has practical interactions in terms of the response
to transplantation, where mice may readily adapt to vascularized graphs,
whereas humans rapidly reject them. This is also felt to be secondary to the
ability of human endothelial cells to present antigen compared with mice.
The mouse and human immune systems are felt to have
diverged 65 mil-lion years ago, although thus far, only 300 genes are felt to
be unique to each spe-cies. The adaptations were in response to various
pathological challenges based on ecological niche. For example, that mice are
closer to the ground would change the exposure and response to microor-ganisms
encountered. Even the differ-ence in life spans would account for the
difference in the immune response. For example, transit times of immune cells
are different between mice and humans and a larger T-cell and B-cell repertoire
must be continued for many years in humans. Humans would encounter more somatic
mutations over time, and greater control of the immune system must be generated
to control for autoimmunity and to con-trol larger, widely varied
antigen-specific clones.
Thus, one can see multiple reasons for often wide
discrepancies between animal models and the human condition, particu-larly in
the response to potential therapeu-tic treatments. Perhaps the focus of effort
and funding should be placed on direct human studies, whether on the molecular,
tissue, or organism level, to unravel the designs of these diseases.
Related Topics