Chapter: Medical Microbiology: An Introduction to Infectious Diseases: Bacterial Structures
Gram Positive Cell Wall - Bacterial Structures
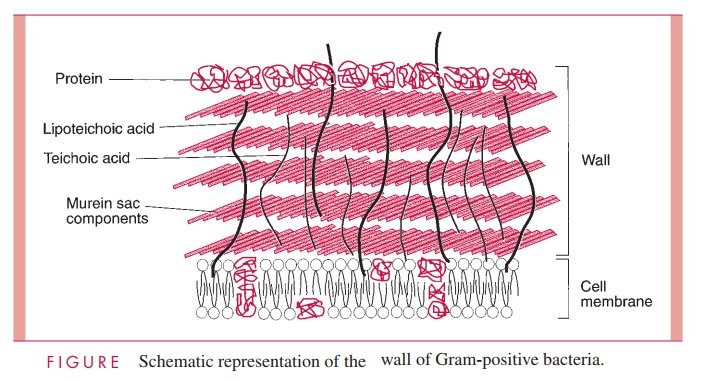
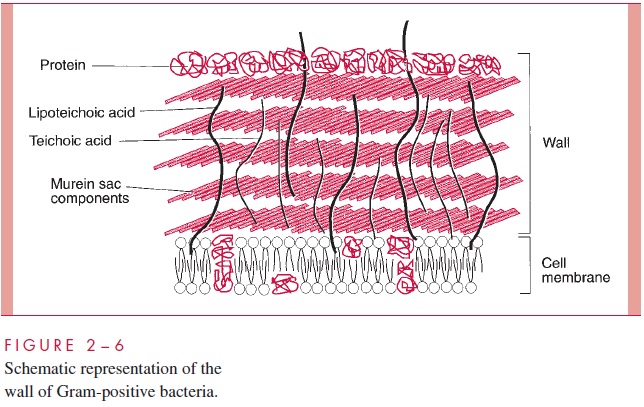
Gram-Positive Cell Wall
The Gram-positive cell wall contains two major components, peptidoglycan and teichoic acids, plus additional carbohydrates and proteins, depending on the species. A general-ized scheme illustrating the arrangement of these components is shown in Figure 2 – 6.
The chief component is murein, a peptidoglycan, which is found nowhere except in prokaryotes. Murein consists of a linear glycan chain of two alternating sugars, N-acetyl-glucosamine (NAG) and N-acetylmuramic acid (NAM), in 1:4 linkages (Fig 2 – 7). Each muramic acid residue bears a tetrapeptide of alternating L- and D-amino acids. Adjacent glycan chains are cross-linked into sheets by peptide bonds between the third amino acid of one tetrapeptide and the terminal D-alanine of another. The same cross-links between other tetrapeptides connect the sheets to form a three-dimensional, rigid matrix. The cross-links involve perhaps one third of the tetrapeptides and may be direct or may in-clude a peptide bridge, as, for example, a pentaglycine bridge in Staphylococcus aureus. The cross-linking extends around the cell, producing a scaffold-like giant molecule, termed the murein sac, or sacculus. Murein is much the same in all bacteria, except that there is diversity in the nature and frequency of the cross-linking bridge and in the nature of the amino acids at positions 2 and 3 of the tetrapeptide.
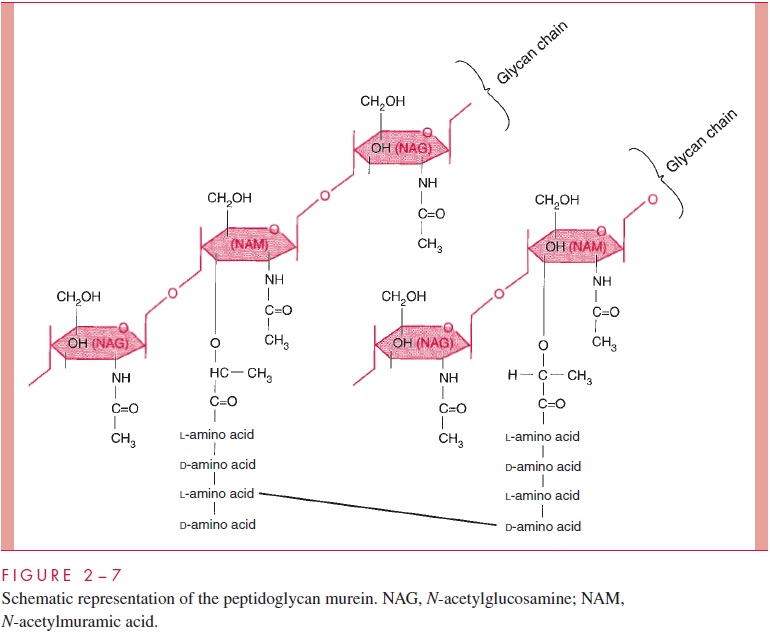
The murein sac derives its great mechanical strength from the fact that it is a single, covalently bonded structure; other features contributing strength are the -1,4 bonds of the polysaccharide backbone, the alternation of D- and L-amino acids in the tetrapeptide, and extensive internal hydrogen bonding. Biological stability is contributed by compo-nents of murein that are not widely distributed in the biological world or in fact are unique to murein. These include muramic acid, D-amino acids, and diaminopimelic acid (an amino acid found in the tetrapeptide of some species). Most enzymes found in mam-malian hosts and other biological systems do not degrade peptidoglycan; one important exception is lysozyme, the hydrolase present in tears and other secretions, which cleaves theβ-1,4 glycosidic bond between muramic acid and glucosamine residues (see Fig 2 – 7). On the other hand, bacteria themselves are rich in hydrolases that degrade peptidoglycan, because the murein sac must be constantly expanded by insertion of new chains as the cell grows and forms a cross-wall preparatory to cell division. As we shall learn, disruption of the fine control that bacteria exert over the activity of these potentially lethal enzymes is the means by which a large number of antibiotics and other chemother-apeutic compounds work .
The role of the murein component of the cell wall in conferring osmotic resistance and shape on the cell is easily demonstrated by removing or destroying it. Treatment of a Gram-positive cell with penicillin (which blocks formation of the tetrapeptide cross-links and activates the cell’s own murein hydrolases) or with lysozyme (which directly hy-drolyzes the glycan chains) destroys the murein sac, and the wall is lost. Prompt lysis of the cell ensues. If the cell is protected from lysis by suspension in a medium approxi-mately isotonic with the cell interior, such as 20% sucrose, the cell becomes round and forms a sphere called a protoplast. Some protoplasts can grow, and their formation from classic bacteria within patients treated with penicillin-type antibiotics (L-forms) has been postulated to account for some persistent infections. Superficially, protoplasts resemble the mollicutes (mycoplasmas) that are naturally wall-less bacteria.
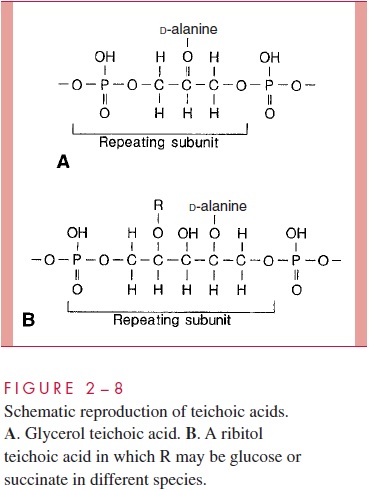
A second component of the Gram-positive cell wall is a teichoic acid. These com-pounds are polymers of either glycerol phosphate or ribitol phosphate, with various sug-ars, amino sugars, and amino acids as substituents (Fig 2 – 8). The lengths of the chain and the nature and location of the substituents vary from species to species and some-times between strains within a species. Up to 50% of the wall may be teichoic acid, some of which is covalently linked to occasional NAM residues of the murein. Of the teichoic acids made of polyglycerol phosphate, much is linked not to the wall but to a glycolipid in the underlying cell membrane. This type of teichoic acid is called lipoteichoic acid and seems to play a role in anchoring the wall to the cell membrane and as an epithelial cell adhesin. Teichoic acids are found only in Gram-positive cells and constitute major antigenic determinants of their cell surface individuality. For example, S. aureus polysac-charide A is a teichoic acid and Enterococcus faecalis group D carbohydrate is a lipotei-choic acid.
Beside the major wall components — murein and teichoic acids — Gram- positive walls usually have lesser amounts of other molecules. Some are polysaccharides, such as the group-specific antigens of streptococci; others are proteins, such as the M protein of group A streptococci. The detailed arrangement of the various antigens in some of the more complex Gram-positive walls is still being worked out, but minor components are thought to protect the peptidoglycan layer from the action of such agents as lysozyme. Some protein components, called adhesins, of the cell wall promote colonization by sticking the bacteria to the surfaces of host cells .
Related Topics