Chapter: Medical Microbiology: An Introduction to Infectious Diseases: Bacterial Structures
Cell Wall - Bacterial Structures
Cell Wall
Internal to the capsule (if one exists) but still outside the cell proper, a rigid cell wall surrounds all eubacterial cells except wall-less bacteria such as the mollicutes (mycoplas-mas) and Chlamydia. The structure and function of the bacterial wall is so distinctive as to constitute a hallmark of the prokaryotes; nothing like it is found elsewhere. Unlike the capsule, which is dispensable for survival outside the body of the host, the wall has vital functions in all environments. It protects the cell from mechanical disruption and from bursting caused by the turgor pressure resulting from the hypertonicity of the cell interior relative to the environment. The wall provides a barrier against certain toxic chemical and biological agents. In some bacterial species, such as Streptococcus , it provides a protection from phagocytosis and helps in the binding to eukaryotic cell hosts. Its form is responsible for the shape of the cell.
Bacterial evolution has led to two major solutions to the challenge of constructing a wall that can protect a minute, fragile cell from chemical and physical assault while still permitting the rapid exchange of nutrients and metabolic byproducts required by rapid growth. Long before these solutions were understood in ultrastructural terms, it was recognized that bacteria could be divided into two groups depending on their reaction to a particular staining procedure devised a century ago by the Danish microbiologist Hans Christian Gram. This procedure, the Gram stain. It depends on the differential ability of ethanol or ethanol–acetone mixtures to extract iodine–crystal violet complexes from bacterial cells. These complexes are readily extracted from one group of bacteria, termed Gram-negative, which can be subsequently stained red with an appropriate counterstain. They are retained by the other, termed Gram-positive, which are thus stained violet by the retained crystal violet. The positive or negative Gram stain response of a cell reflects which of the two types of wall it possesses.
Virtually all of the eubacteria with walls can be assigned a Gram response. However, the few exceptions include some medically important organisms. For example, the my-cobacteria (eg, Mycobacterium tuberculosis, the causative agent of tuberculosis) are Gram positive on the basis of their wall structure but fail to stain because of interference by spe-cial lipids present in their walls. Most spirochetes, including Treponema pallidum (the causative agent of syphilis), although Gram negative by structure, are too thin to be re-solved in the light microscope when stained by simple stains.
Bacteria without walls, whether natural forms (the mollicutes or mycoplasmas) or artificial products of procedures that remove the wall, exhibit a Gram-negative staining response. Furthermore, some bacteria that are Gram positive on the basis of wall structure and staining response may lose this property and appear Gram negative if they have been held under nongrowing conditions. These examples emphasize that being Gram positive is a distinct property that can be temporarily lost because it depends on the integrity of the cell wall; on the other hand, a Gram-negative bacterial cell does not have a staining property to lose.
Gram-Positive Cell Wall
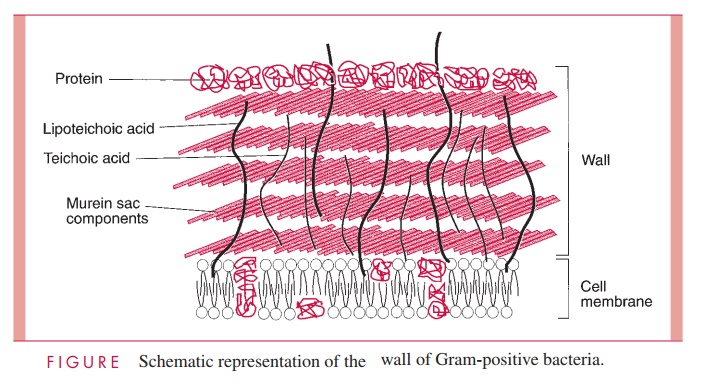
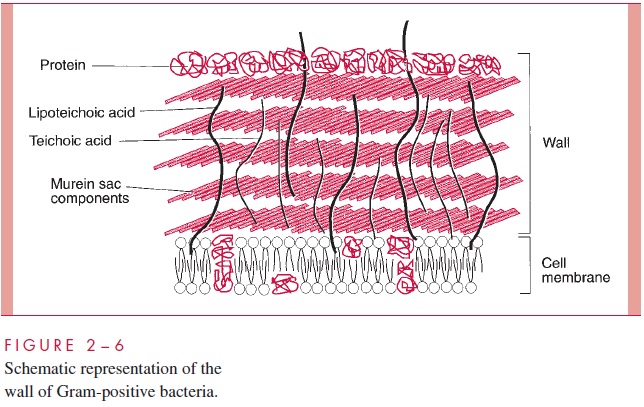
The Gram-positive cell wall contains two major components, peptidoglycan and teichoic acids, plus additional carbohydrates and proteins, depending on the species. A general-ized scheme illustrating the arrangement of these components is shown in Figure 2 – 6.
The chief component is murein, a peptidoglycan, which is found nowhere except in prokaryotes. Murein consists of a linear glycan chain of two alternating sugars, N-acetyl-glucosamine (NAG) and N-acetylmuramic acid (NAM), in 1:4 linkages (Fig 2 – 7). Each muramic acid residue bears a tetrapeptide of alternating L- and D-amino acids. Adjacent glycan chains are cross-linked into sheets by peptide bonds between the third amino acid of one tetrapeptide and the terminal D-alanine of another. The same cross-links between other tetrapeptides connect the sheets to form a three-dimensional, rigid matrix. The cross-links involve perhaps one third of the tetrapeptides and may be direct or may in-clude a peptide bridge, as, for example, a pentaglycine bridge in Staphylococcus aureus. The cross-linking extends around the cell, producing a scaffold-like giant molecule, termed the murein sac, or sacculus. Murein is much the same in all bacteria, except that there is diversity in the nature and frequency of the cross-linking bridge and in the nature of the amino acids at positions 2 and 3 of the tetrapeptide.
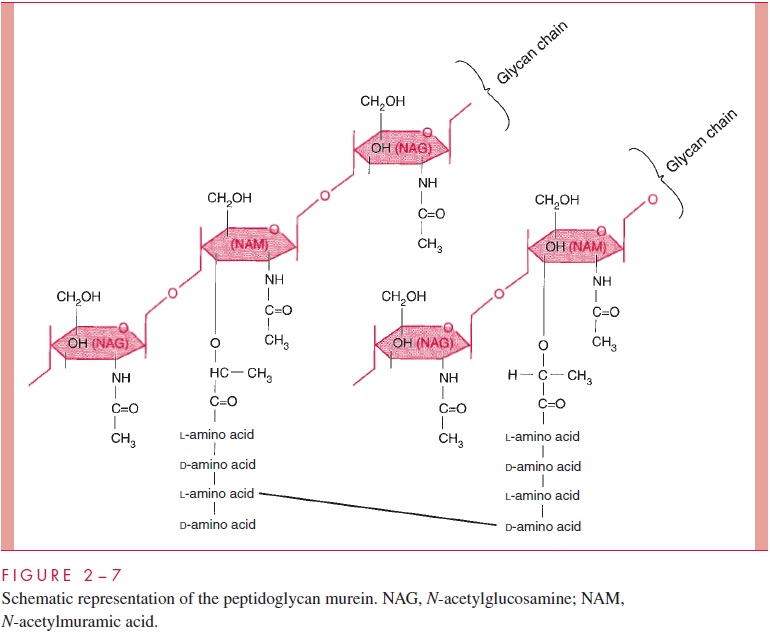
The murein sac derives its great mechanical strength from the fact that it is a single, covalently bonded structure; other features contributing strength are the -1,4 bonds of the polysaccharide backbone, the alternation of D- and L-amino acids in the tetrapeptide, and extensive internal hydrogen bonding. Biological stability is contributed by compo-nents of murein that are not widely distributed in the biological world or in fact are unique to murein. These include muramic acid, D-amino acids, and diaminopimelic acid (an amino acid found in the tetrapeptide of some species). Most enzymes found in mam-malian hosts and other biological systems do not degrade peptidoglycan; one important exception is lysozyme, the hydrolase present in tears and other secretions, which cleaves the β-1,4 glycosidic bond between muramic acid and glucosamine residues (see Fig 2 – 7). On the other hand, bacteria themselves are rich in hydrolases that degrade peptidoglycan, because the murein sac must be constantly expanded by insertion of new chains as the cell grows and forms a cross-wall preparatory to cell division. As we shall learn, disruption of the fine control that bacteria exert over the activity of these potentially lethal enzymes is the means by which a large number of antibiotics and other chemother-apeutic compounds work .
The role of the murein component of the cell wall in conferring osmotic resistance and shape on the cell is easily demonstrated by removing or destroying it. Treatment of a Gram-positive cell with penicillin (which blocks formation of the tetrapeptide cross-links and activates the cell’s own murein hydrolases) or with lysozyme (which directly hy-drolyzes the glycan chains) destroys the murein sac, and the wall is lost. Prompt lysis of the cell ensues. If the cell is protected from lysis by suspension in a medium approxi-mately isotonic with the cell interior, such as 20% sucrose, the cell becomes round and forms a sphere called a protoplast. Some protoplasts can grow, and their formation from classic bacteria within patients treated with penicillin-type antibiotics (L-forms) has been postulated to account for some persistent infections. Superficially, protoplasts resemble the mollicutes (mycoplasmas) that are naturally wall-less bacteria.
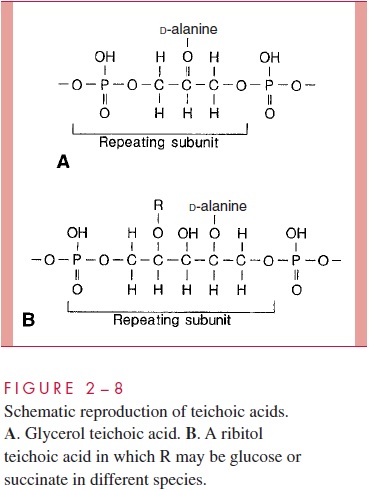
A second component of the Gram-positive cell wall is a teichoic acid. These com-pounds are polymers of either glycerol phosphate or ribitol phosphate, with various sug-ars, amino sugars, and amino acids as substituents (Fig 2 – 8). The lengths of the chain and the nature and location of the substituents vary from species to species and some-times between strains within a species. Up to 50% of the wall may be teichoic acid, some of which is covalently linked to occasional NAM residues of the murein. Of the teichoic acids made of polyglycerol phosphate, much is linked not to the wall but to a glycolipid in the underlying cell membrane. This type of teichoic acid is called lipoteichoic acid and seems to play a role in anchoring the wall to the cell membrane and as an epithelial cell adhesin. Teichoic acids are found only in Gram-positive cells and constitute major antigenic determinants of their cell surface individuality. For example, S. aureus polysac-charide A is a teichoic acid and Enterococcus faecalis group D carbohydrate is a lipotei-choic acid.
Beside the major wall components — murein and teichoic acids — Gram- positive walls usually have lesser amounts of other molecules. Some are polysaccharides, such as the group-specific antigens of streptococci; others are proteins, such as the M protein of group A streptococci. The detailed arrangement of the various antigens in some of the more complex Gram-positive walls is still being worked out, but minor components are thought to protect the peptidoglycan layer from the action of such agents as lysozyme. Some protein components, called adhesins, of the cell wall promote colonization by sticking the bacteria to the surfaces of host cells .
Gram-Negative Cell Wall
The second kind of cell wall found in bacteria, the Gram-negative cell wall, is depicted in Figure 2 – 9. Except for the presence of murein, there is little chemical resemblance to cell walls of Gram-positive bacteria, and the architecture is fundamentally different. In Gram-negative cells, the amount of murein has been greatly reduced, with some of it forming a single-layered sheet around the cell and the rest forming a gel-like substance, the periplasmic gel, with little cross-linking. External to this periplasm is an elaborate outer membrane.
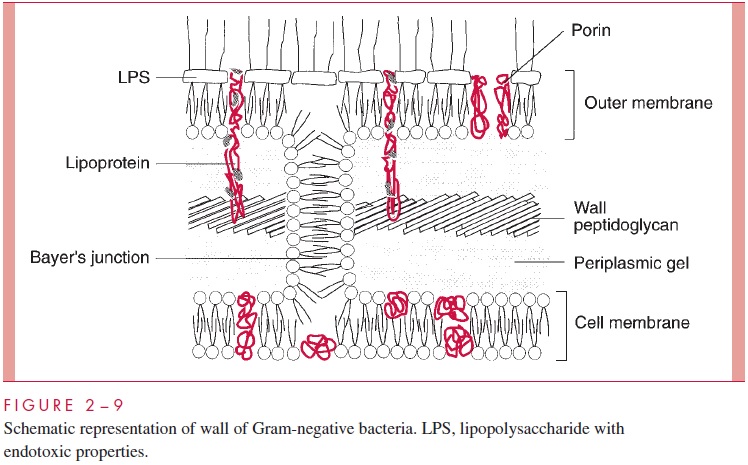
Historically, the cell wall was regarded as the structure external to the cell membrane (excluding the capsule), and for Gram-positive bacteria this conception is certainly appro-priate. Examination of Figures 2 – 5 and 2 – 9 shows the dilemma in applying the same term to the Gram-negative envelope. There is some reason to apply the same definition used for the Gram-positive situation, in which case the cell wall of Gram-negative bacte-ria consists of periplasm with its murein sac plus the outer membrane. This convention is used in Table 2 – 1. An alternative convention is to consider that the cell wall of Gram-negative bacteria is simply the structure chemically most like the Gram-positive wall, namely, the thin murein sac, with perhaps its attendant periplas-mic gel. The student will quickly realize the underlying truth that cell wall is not a very satisfying term. Some microbiologists use cell envelope and envelope layers and avoid using the term cell wall altogether for Gram-negative bacteria.
Earlier electron micrographs had suggested that the small amount of murein in Gram-negative cells, such as Escherichia coli, formed a single sheet around the cell, and that this murein sac was floating in a space, the periplasmic space, containing a fairly concen-trated solution of proteins and oligosaccharides. Recent evidence modifies this picture and indicates that the “space” is a gel formed by murein peptidoglycan chains with little or no cross-linking.
Whatever its precise nature, the periplasm contains a murein sac, with a unit peptido-glycan structure quite similar to that in Gram-positive cells. Despite its reduced extent in the Gram-negative wall, the murein sac still is responsible for the shape of the cell and is vital for its integrity. As in the case of Gram-positive cells, removing or damaging the peptidoglycan layer leads to cell lysis. If the cells are protected from osmotic lysis dur-ing lysozyme or penicillin treatment, they assume a spherical shape. Because such spheres cannot be totally stripped of wall material, they are calledspheroplasts, in con-trast to the protoplasts formed from Gram-positive cells. Spheroplasts of some species can multiply.
The proteins in solution in the periplasm consist of enzymes with hydrolytic functions (such as alkaline phosphatase), sometimes antibiotic-inactivating enzymes, and various binding proteins with roles in chemotaxis and in the active transport of solutes into the cell . Oligosaccharides secreted into the periplasm in response to external conditions serve to create an osmotic pressure buffer for the cell.
The periplasm is an intermembrane structure, lying between the cell membrane (dis-cussed later) and a special membrane unique to Gram-negative cells, the outer membrane. This has an overall structure similar to most biological membranes with two opposing phospholipid – protein leaflets. However, in terms of its composition, the outer membrane is unique in all biology. Its inner leaflet consists of ordinary phospholipids, but these are replaced in the outer leaflet by a special molecule called lipopolysaccharide (LPS), which is extremely toxic to humans and other animals, and is called an endotoxin. Even in minute amounts, such as the amount released to the circulation during the course of a Gram-negative infection, this substance can produce a fever and shock syndrome calle Gram-negative shock, or endotoxic shock.
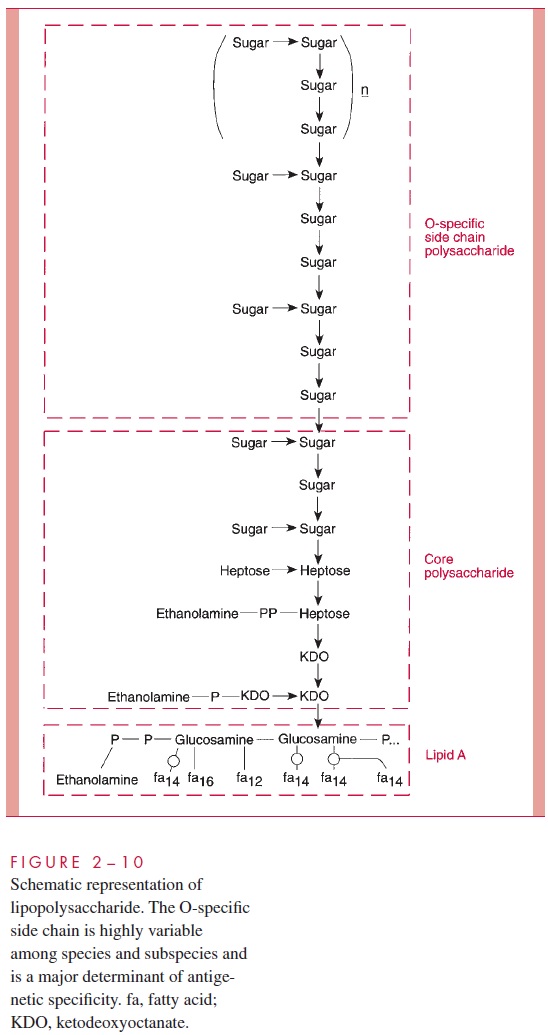
LPS consists of a toxic lipid A (a phospholipid containing glucosamine rather than glycerol), a core polysaccharide (containing some unusual carbohydrate residues and fairly constant in structure among related species of bacteria), and O antigen polysac-charide side chains (Fig 2 – 10). The last component constitutes the major surface anti-gen of Gram-negative cells (which, it is recalled, lack teichoic acids).
The presence of LPS in the outer leaflet of the outer membrane results in the cover-ing of Gram-negative cells by a wall that should block the passage of virtually every organic molecule into the cell. Hydrophobic molecules (such as some antibiotics) would be blocked by the hydrophilic layer of O antigen; hydrophilic solutes, including most nutrients, such as sugars and amino acids, would face the barrier created by the
Clearly this is a trade-off that cannot be made; the Gram-negative cell, for whatever benefit is afforded by possessing a wall with an outer membrane, must make provision for the rapid entry of nutrients. Active transport is part of the solution, and a particular structural feature of the outer membrane contributes another part. Special proteins, called porins or matrixproteins, form pores through the outer membrane that make it possible for hydrophilicsolute molecules of molecular weight less than about 800 to diffuse through it and into the periplasm.
The outer membrane does not contain the variety of proteins present in the cell membrane, but those that are present are quite abundant. In addition to the porins, there is a protein called Braun’s lipoprotein or murein lipoprotein, which is probably the most abundant outer membrane protein in Gram-negative cells, such as E. coli. This protein is covalently attached at its amino end to a lipid embedded in the outer mem-brane. About one third of these lipoprotein molecules are covalently attached at their carboxyl end to the third amino acid in the murein tetrapeptide. It is believed that this forms the major attachment of the murein layer to the outer membrane of the wall in E. coli (see Fig 2 – 9).
The innermost leaflet may well be contiguous in places with the outermost leaflet of the cell membrane (see Fig 2 – 9), because, at least under the electron microscope, prepa-rations of the outer membrane and the cell membrane can be seen to adhere to each other at zones of adhesion (also calledBayer’s junctions). Other zones of adhesion girding the whole circumference of E. coli and related species have been postulated. Because these annular rings tend to form about the cell division septum, they have been called periseptal annuli. Their existence is still being examined.
In evolving a cell wall containing an outer membrane, Gram-negative bacteria have succeeded in (1) creating the periplasm, which holds digestive and protective enzymes and proteins important in transport and chemotaxis; (2) presenting an outer surface with strong negative charge, which is important in evading phagocytosis and the action of complement; and (3) providing a permeability barrier against such dangerous molecules as host lysozyme, β-lysin, bile salts, digestive enzymes, and many antibiotics.
Related Topics