Chapter: Biochemistry: Glycolysis
Glyceraldehyde-3-Phosphate Is Converted to Pyruvate
Glyceraldehyde-3-Phosphate Is
Converted to Pyruvate
At this
point, a molecule of glucose (a six-carbon compound) that enters the pathway
has been converted to two molecules of glyceraldehyde-3-phosphate. We have not
seen any oxidation reactions yet, but now we shall encounter them. Keep in mind
that in the rest of the pathway two molecules of each of the three-carbon
compounds take part in every reaction for each original glucose molecule.
Figure 17.7 summarizes the second part of the pathway, which is often referred
to as the payoff phase of glycolysis,
since ATP is produced instead of used in this phase.
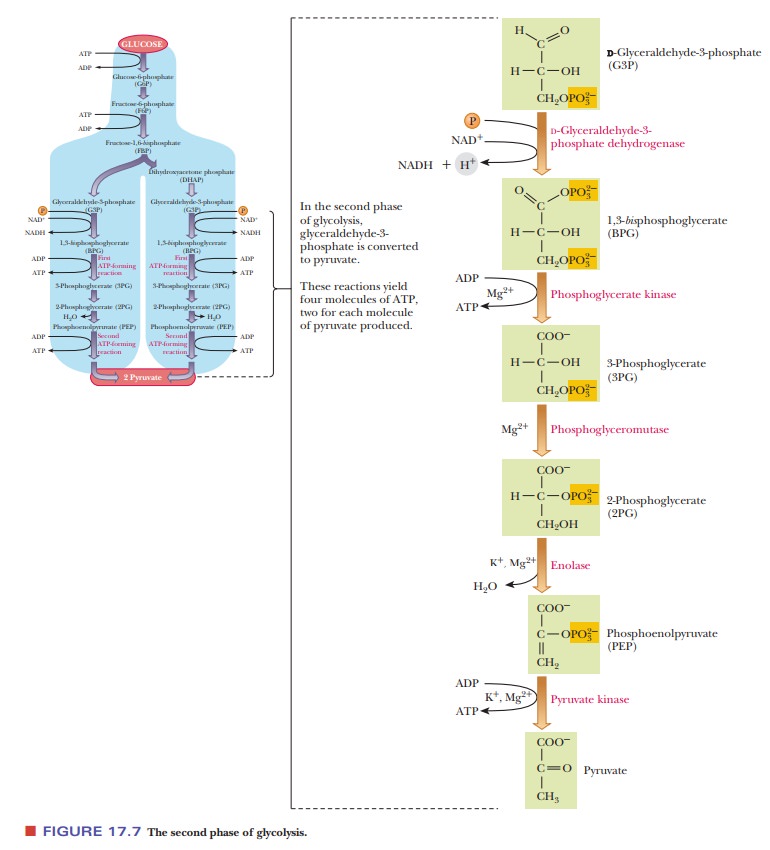
What reactions convert glyceraldehyde-3-phosphate to pyruvate?
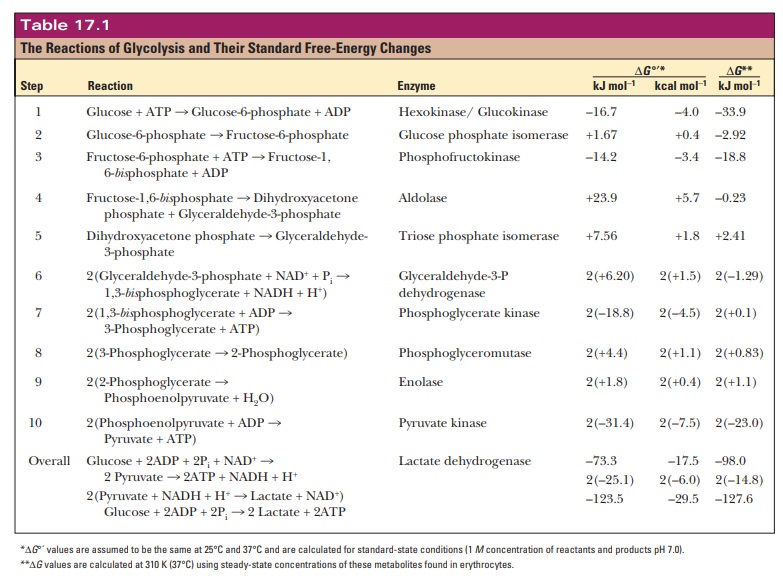
Step 6. Glyceraldehyde-3-phosphate is oxidized to 1,3-bisphosphoglycerate.
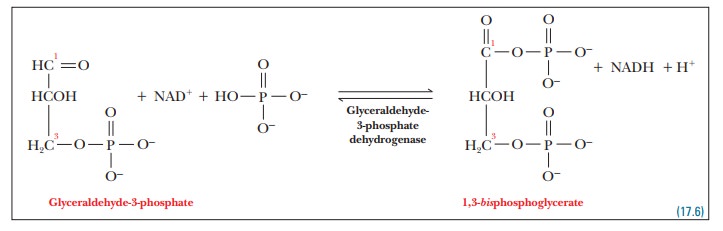
This
reaction, the characteristic reaction
of glycolysis, should be looked at more closely. It involves the addition of a
phosphate group to glyceraldehyde- 3-phosphate as well as an electron-transfer
reaction, from glyceraldehyde-3- phosphate to NAD+. We will simplify
the discussion by considering the two parts separately.
The half
reaction of oxidation is that of an aldehyde to a carboxylic acid group, in
which water can be considered to take part in the reaction.
RCHO + H2O
- > RCOOH + 2H+ + 2e–
The half
reaction of reduction is that of NAD+ to NADH.
NAD+
+ 2H+ + 2e– -
> NADH + H+
The
overall redox reaction is thus
RCHO + H
O + NAD+3 RCOOH + H+ + NADH
in which
R indicates the portions of the molecule other than the aldehyde and carboxylic
acid groups, respectively. The oxidation reaction is exergonic under standard
conditions ( ∆G°' = –43.1 kJ mol–1 = –10.3 kcal mol–1),
but oxidation is only part of the overall reaction.
The
phosphate group that is linked to the carboxyl group does not form an ester,
since an ester linkage requires an alcohol and an acid. Instead, the car-
boxylic acid group and phosphoric acid form a mixed anhydride of two acids by
loss of water, 3-Phosphoglycerate + Pi - > 1,3-bisphosphoglycerate + H2O in
which the substances involved in the reaction are in the ionized form
appropriate at pH 7. Note that ATP and ADP do not appear in the equation. The
source of the phosphate group is phosphate ion itself, rather than ATP. The
phosphorylation reaction is endergonic under standard conditions ( ∆G°'
= 49.3 kJ mol–1 = 11.8 kcal mol–1).
The
overall reaction, including electron transfer and phosphorylation, is
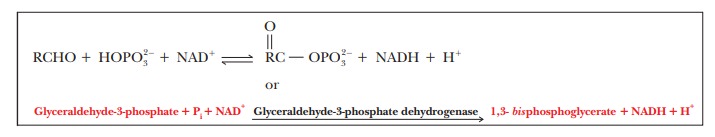
Let’s show the two reactions that make up this reaction.
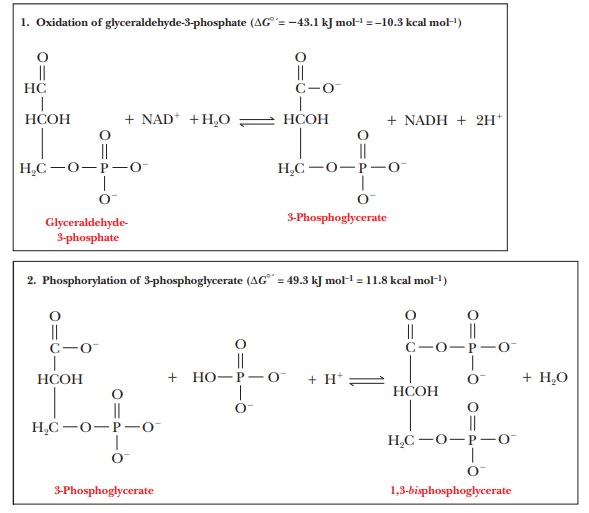
The
standard free-energy change for the overall reaction is the sum of the values
for the oxidation and phosphorylation reactions. The overall reaction is not
far from equilibrium, being only slightly endergonic.
∆G°' overall =∆G°'
oxidation + ∆G°' phosphorylation
= (–43.1
kJ mol–1) + (49.3 kJ mol–1)
= 6.2 kJ
mol–1 = 1.5 kcal mol–1
This
value of the standard free-energy change is for the reaction of one mole of
glyceraldehyde-3-phosphate; the value must be multiplied by 2 to get the value
for each mole of glucose ( ∆G°' =
12.4 kJ mol–1 = 3.0 kcal mol–1). The G under cellular conditions is slightly negative (–1.29 kJ mol–1
or –0.31 kcal mol–1) (Table 17.1). The enzyme that catalyzes the
conversion of glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate is glyceraldehyde-3-phosphate
dehydrogenase. This enzyme is one of a class of similar enzymes, the
NADH-linked dehydrogenases. The structures of a number of dehydrogenases of
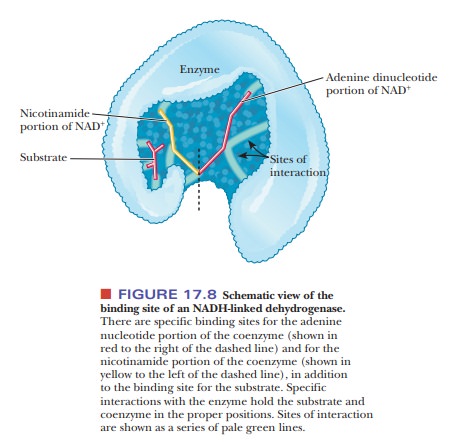
this type have been studied via X-ray crystallography. The overall structures
are not strikingly similar, but the structure of the binding site for NADH is
quite similar in all these enzymes (Figure 17.8). (The oxidizing agent is NAD+;
both oxidized and reduced forms of the coenzyme bind to the enzyme.) One
portion of the binding site is specific for the nicotinamide ring, and one
portion is specific for the adenine ring.
The
molecule of glyceraldehyde-3-phosphate dehydrogenase is a tetramer, consisting
of four identical subunits. Each subunit binds one molecule of NAD+,
and each subunit contains an essential cysteine residue. A thioester involving
the cysteine residue is the key intermediate in this reaction. In the
phosphoryla-tion step, the thioester acts as a high-energy intermediate.
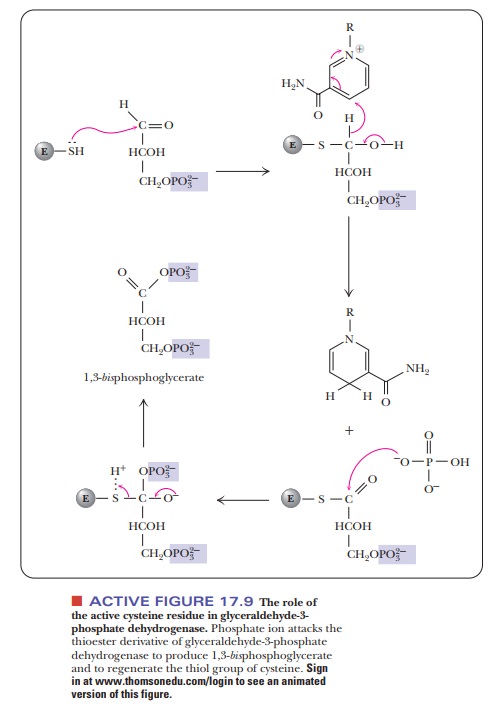
Phosphate
ion attacks the thioester, forming a mixed anhydride of the carboxylic and
phosphoric acids, which is also a high-energy compound (Figure 17.9). This
compound is 1,3-bisphosphoglycerate,
the product of the reaction. Production of ATP requires a high-energy compound
as starting material. The 1,3-bisphosphoglycerate
fulfills this requirement and transfers a phosphate group to ADP in a highly
exergonic reaction (i.e., it has a high phosphate-group transfer potential).
Step 7.The next step is one of the two
reactions in which ATP is produced byphosphorylation of ADP.
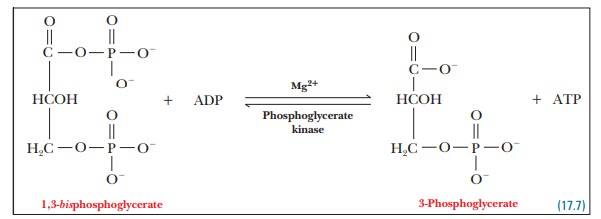
The
enzyme that catalyzes this reaction is phosphoglycerate
kinase. By now the term kinase
should be familiar as the generic name for a class of ATP-dependent
phosphate-group transfer enzymes. The most striking feature of the reaction has
to do with energetics of the phosphate-group transfer. In this step in
glycolysis, a phosphate group is transferred from 1,3-bisphosphoglycerate to a molecule of ADP, producing ATP, the first
of two such reactions in the gly-colytic pathway. We already mentioned that
1,3-bisphosphoglycerate can easily
transfer a phosphate group to other substances. Note that a substrate, namely
1,3-bisphosphoglycerate, has
transferred a phosphate group to ADP. This trans-fer is typical of substrate-level phosphorylation. It is
to be distinguished from oxidative phosphorylation, in which transfer of
phosphate groups is linked to electron-transfer reactions in which oxygen is
the ultimate electron acceptor. The only requirement for substrate-level
phosphor-ylation is that the standard free energy of the hydrolysis reaction is
more nega-tive than that for hydrolysis of the new phosphate compound being
formed. Recall that the standard free energy of hydrolysis of 1,3-bisphosphoglycerate is –49.3 kJ mol–1.
We have already seen that the standard free energy of hydrolysis of ATP is
–30.5 kJ mol–1, and we must change the sign of the free-energy
change when the reverse reaction occurs:
ADP + Pi
+ H+ - > ATP + H2O
∆G°' = 30.5 kJ mol–1= 7.3 kcal mol–1
The net
reaction is
1,3-bisphosphoglycerate + ADP - > 3-Phosphoglycerate
+ ATP
∆G°' = –49.3 kJ mol–1+ 30.5 kJ mol–1=
–18.8 kJ mol–1= –4.5 kcal mol–1
Two
molecules of ATP are produced by this reaction for each molecule of glucose
that enters the glycolytic pathway. In the earlier stages of the pathway, two
molecules of ATP were invested to produce fructose-1,6-bisphosphate, and now they have been recovered. At this point, the
balance of ATP use and pro-duction is exactly even. The next few reactions will
bring about the production of two more molecules of ATP for each original
molecule of glucose, leading to the net gain of two ATP molecules in
glycolysis.
Step 8.The phosphate group is transferred
from carbon - > to carbon 2 of theglyceric acid backbone, setting the stage
for the reaction that follows.
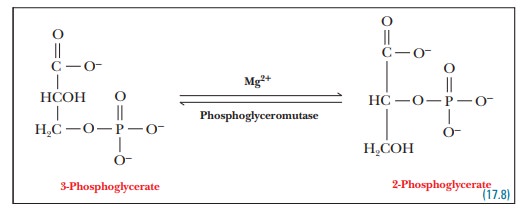
The
enzyme that catalyzes this reaction is phosphoglyceromutase.
Step 9.The 2-phosphoglycerate molecule loses
one molecule of water,producing phosphoenolpyruvate. This reaction does not
involve electron transfer; it is a dehydration reaction. Enolase, the enzyme that catalyzes this reaction, requires Mg2+
as a cofactor. The water molecule that is eliminated binds to Mg2+
in the course of the reaction.
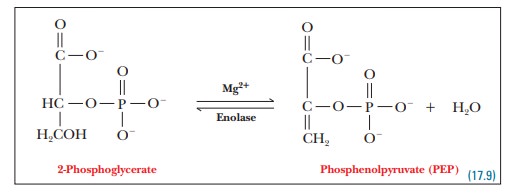
Step 10.Phosphoenolpyruvate transfers its
phosphate group to ADP, pro-ducing ATP and pyruvate.
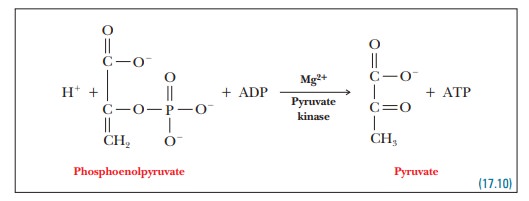
The
double bond shifts to the oxygen on carbon 2 and a hydrogen shifts to carbon
Phosphoenolpyruvate
is a high-energy compound with a high phosphate-group transfer potential. The
free energy of hydrolysis of this compound is more negative than that of ATP
(–61.9 kJ mol–1 versus –30.5 kJ mol–1, or –14.8 kcal mol–1
versus –7.3 kcal mol–1). The reaction that occurs in this step can
be considered to be the sum of the hydrolysis of phosphoenolpyruvate and the
phosphorylation of ADP. This reaction is another example of substrate-level
phosphorylation.
Phosphoenolpyruvate
- > Pyruvate + Pi
∆G°' = –61.9 kJ mol–1= –14.8 kcal mol–1ADP
+ Pi - > ATP
∆G°' = 30.5 kJ mol–1= 7.3 kcal mol–1
The net
reaction is Phosphoenolpyruvate + ADP - > Pyruvate + ATP
∆G°' = –31.4 kJ mol–1= –7.5 kcal mol–1
Since
two moles of pyruvate are produced for each mole of glucose, twice as much
energy is released for each mole of starting material.
Pyruvate kinase is the enzyme that catalyzes this reaction.
Like phospho-fructokinase, it is an allosteric enzyme consisting of four
subunits of two dif-ferent types (M and L), as we saw with phosphofructokinase.
Pyruvate kinase is inhibited by ATP. The conversion of phosphoenolpyruvate to
pyruvate slows down when the cell has a high concentration of ATP—that is to
say, when the cell does not have a great need for energy in the form of ATP.
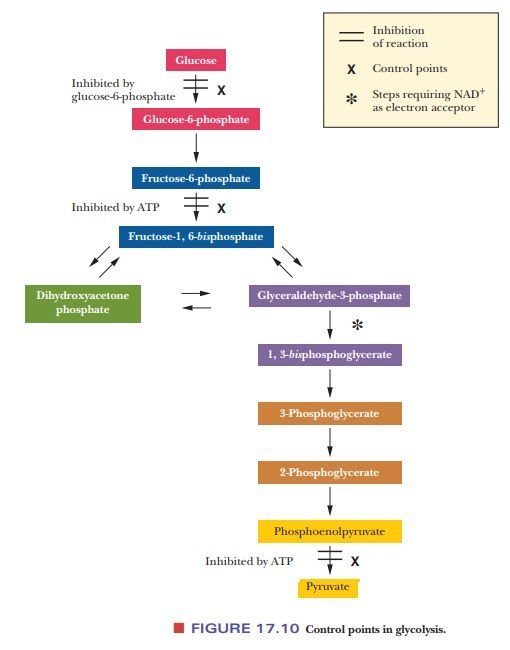
Where are the control points in the glycolytic pathway?
One of the most important questions that we can ask about any
metabolic pathway is, at which points is control exercised? Pathways can be
“shut down” if an organism has no immediate need for their products, which
saves energy for the organism. In glycolysis, three reactions are control
points. The first is the reaction of glucose to glucose-6-phosphate, catalyzed
by hexokinase; the second, which is the production of fructose-1,6-bisphosphate, is catalyzed by
phosphofructokinase; and the last is the reaction of PEP to pyruvate, catalyzed
by pyruvate kinase (Figure 17.10). It is frequently observed that control is
exercised near the start and end of a pathway, as well as at points involving
key intermediates such as fructose-1,6-bisphosphate.
When we have learned more about the metabolism of carbohydrates, we can return
to the role of phosphofructokinase and fructose-1,6-bisphosphate in the regulation of several pathways of carbohydrate
metabolism.
Summary
In the final stages of glycolysis, two molecules of pyruvate are
produced for each molecule of glucose that entered the pathway.
These reactions involve electron transfer (oxidation–reduction) and
the net production of two ATP for each glucose.
There are three control points in the glycolytic pathway.
Related Topics