Chapter: Biochemistry: Glycolysis
Conversion of Six-Carbon Glucose toThree-Carbon Glyceraldehyde-3-Phosphate
Conversion of Six-Carbon Glucose
toThree-Carbon Glyceraldehyde-3-Phosphate
The first steps of the glycolytic pathway prepare for the electron
transfer and the eventual phosphorylation of ADP; these reactions make use of
the free energy of hydrolysis of ATP. Figure 17.3 summarizes this part of the
pathway, which is often called the preparation
phase of glycolysis.
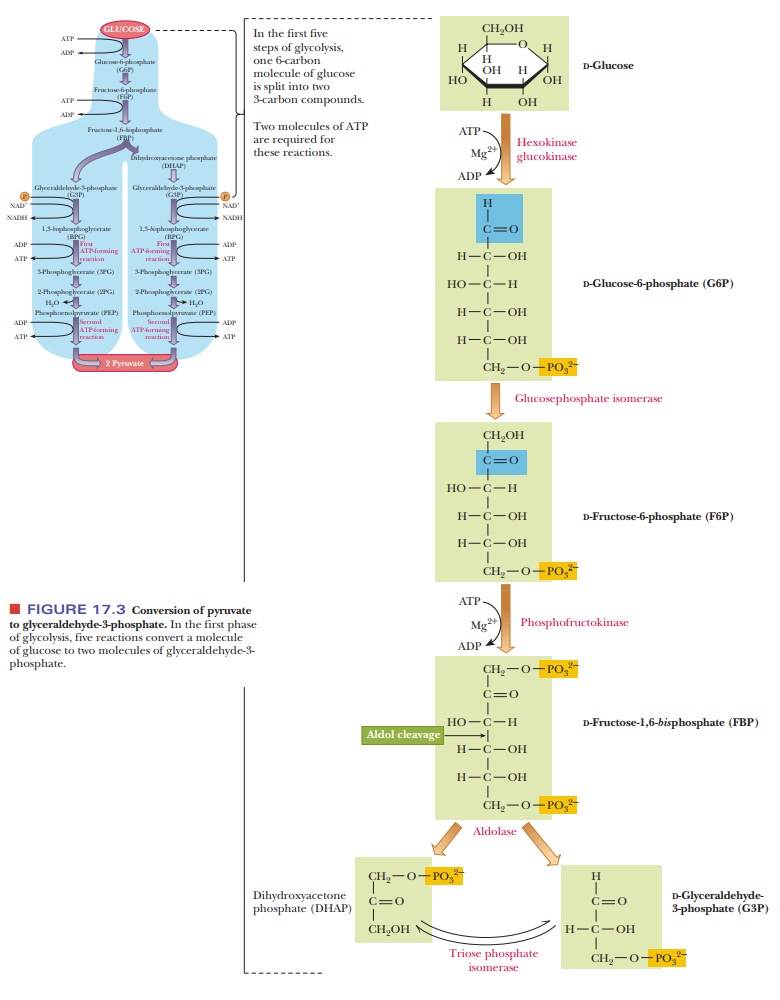
What reactions convert glucose-6-phosphate to glyceraldehyde-3-phosphate?
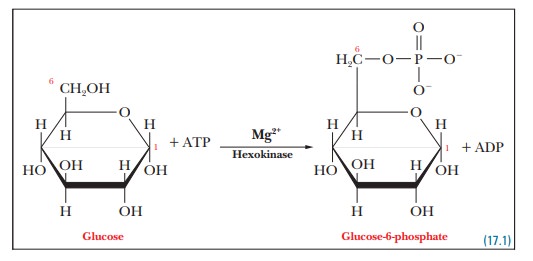
Step 1.Glucose is phosphorylated to give
glucose-6-phosphate. Thephosphorylation of glucose is an endergonic reaction.
Glucose
+ Pi - > Glucose-6-phosphate + H2O
∆G°' = 13.8 kJ mol–1= 3.3 kcal mol–1
The
hydrolysis of ATP is exergonic.
ATP + H2O
- > ADP + Pi
∆G°' = –30.5 kJ mol–1= –7.3 kcal mol–1
These
two reactions are coupled, so the overall reaction is the sum of the two and is
exergonic.
Glucose
+ ATP - > Glucose-6-phosphate + ADP
∆G°' = (13.8 + –30.5) kJ mol–1= –16.7
kJ mol–1= –4.0 kcal mol–1
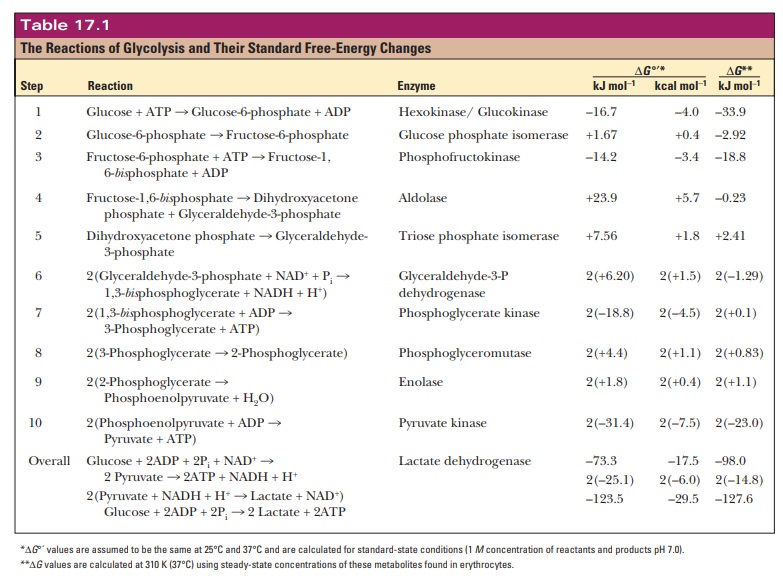
Recall that ∆G°' is calculated under standard states with the concentration of all reactants and products at 1 M except hydrogen ion. If we look at the actual ΔΓGin the cell, the number varies depending on cell type and metabolic state,but a typical value for this reaction is –33.9 kJ mol–1 or –8.12 kcal mol–1. Thus the reaction is typically even more favorable under cellular conditions. Table 17.1 gives the ∆G°' and G values for all the reactions of anaerobic glycolysis in erythrocytes.
This
reaction illustrates the use of chemical energy originally produced by the
oxidation of nutrients and ultimately trapped by phosphorylation of ADP to ATP.
Recall that ATP does not represent stored energy, just as an electric current
does not represent stored energy. The chemical energy of nutrients is released
by oxidation and is made available for immediate use on demand by being trapped
as ATP.
The
enzyme that catalyzes this reaction is hexokinase.
The term kinase is applied to the
class of ATP-dependent enzymes that transfer a phosphate group from ATP to a
substrate. The substrate of hexokinase is not necessarily glucose; rather, it
can be any one of a number of hexoses, such as glucose, fructose, and mannose.
Glucose-6-phosphate inhibits the activity of hexokinase; this is a control
point in the pathway. Some organisms or tissues contain multiple isozymes of
hexokinase. One isoform of hexokinase found in the human liver, called
glucokinase, lowers blood glucose levels after one has eaten a meal. Liver
glucokinase requires a much higher substrate level to achieve saturation than
hexokinase does. Because of this, when glucose levels are high, the liver can
metabolize glucose via glycolysis preferentially over the other tissues. When
glu-cose levels are low, hexokinase is still active in all tissues.
A large
conformational change takes place in hexokinase when substrate is bound. It has
been shown by X-ray crystallography that, in the absence of substrate, two
lobes of the enzyme that surround the binding site are quite far apart. When
glucose is bound, the two lobes move closer together, and the glu-cose becomes
almost completely surrounded by protein (Figure 17.4).

This
type of behavior is consistent with the induced-fit theory of enzyme action. In
all kinases for which the structure is known, a cleft closes when substrate is
bound.
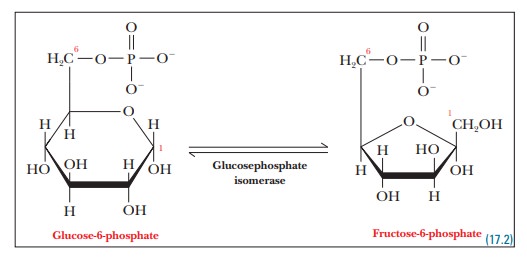
Step 2.Glucose-6-phosphate isomerizes to
give fructose-6-phosphate.Glucosephosphate
isomerase is the enzyme that catalyzes this reaction. TheC-1 aldehyde group
of glucose-6-phosphate is reduced to a hydroxyl group, and the C-2 hydroxyl
group is oxidized to give the ketone group of fructose-6-phosphate, with no net
oxidation or reduction. (Recall that glucose is an aldose, a sugar whose
open-chain, noncyclic structure contains an aldehyde group, while fructose is a
ketose, a sugar whose corresponding structure contains a ketone group.) The
phosphorylated forms, glucose-6-phosphate and fructose-6-phosphate, are an
aldose and a ketose, respectively.
Step 3.Fructose-6-phosphate is further
phosphorylated, producing fructose- 1,6-bisphosphate.
As in
the reaction in Step 1, the endergonic reaction of phosphorylation of
fructose-6-phosphate is coupled to the exergonic reaction of hydrolysis of ATP,
and the overall reaction is exergonic. See Table 17.1.
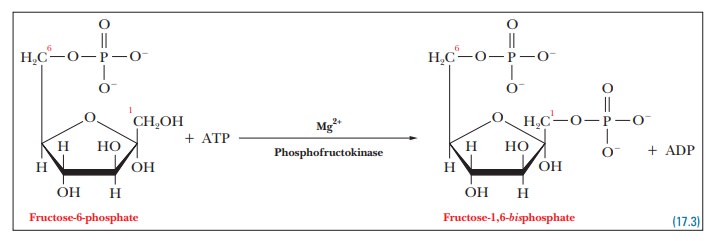
The
reaction in which fructose-6-phosphate is phosphorylated to give fructose-1,6-bisphosphate is the one in which the
sugar is committed to gly-colysis. Glucose-6-phosphate and fructose-6-phosphate
can play roles in other pathways, but fructose-1,6-bisphosphate does not. After fructose-1,6-bisphos-phate is formed from the original sugar, no other pathways
are available, and the molecule must undergo the rest of the reactions of
glycolysis. The phos-phorylation of fructose-6-phosphate is highly exergonic
and irreversible, and phosphofructokinase,
the enzyme that catalyzes it, is the key regulatory enzymein glycolysis.
Phosphofructokinase
is a tetramer that is subject to allosteric feedback regu-lation of the type we
discussed. There are two types of subunits, designated M and L, that can
combine into tetramers to give different per-mutations (M4, M3L,
M2L2, ML3, and L4). These
combinations of subunits are referred to as isozymes, and they have subtle physical and kinetic differences
(Figure 17.5). The subunits differ slightly in amino acid composition, so the
two isozymes can be separated from each other by electrophoresis. The
tetrameric form that occurs in muscle is designated M4, while that
in liver is designated L4. In red blood cells, several of the
combinations can be found. Individuals who lack the gene that directs the
synthesis of the M form of the enzyme can carry on glycolysis in their livers
but experience muscle weakness because they lack the enzyme in muscle.
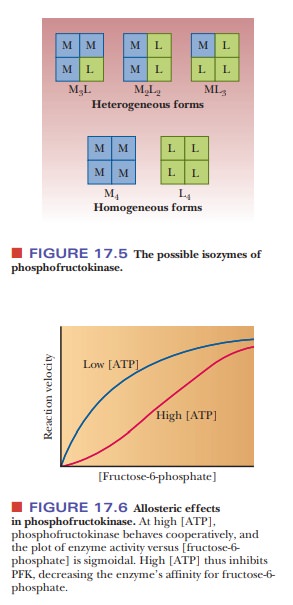
When the
rate of the phosphofructokinase reaction is observed at varying concentrations
of substrate (fructose-6-phosphate), the sigmoidal curve typical of allosteric
enzymes is obtained. ATP is an allosteric effector in the reaction. High levels
of ATP depress the rate of the reaction, and low levels of ATP stimulate the
reaction (Figure 17.6). When there is a high level of ATP in the cell, a good
deal of chemical energy is immediately available from hydrolysis of ATP. The
cell does not need to metabolize glucose for energy, so the presence of ATP
inhibits the glycolytic pathway at this point. There is also another, more
potent, allosteric effector of phosphofructokinase. This effector is
fructose-2,6-bisphosphate; we shall
discuss its mode of action when we consider general control mechanisms in
carbohydrate metabolism.
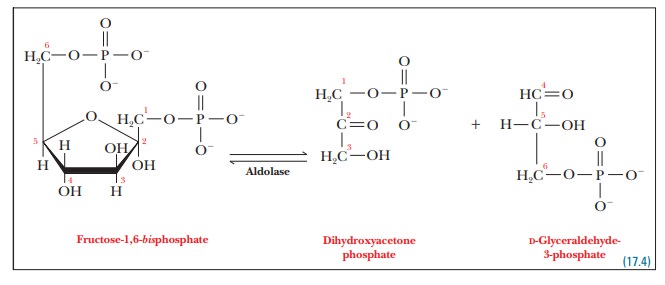
Step 4.Fructose-1,6-bisphosphate is split into two three-carbon fragments. Thecleavage
reaction here is the reverse of an aldol condensation; the enzyme that
catalyzes it is called aldolase. In
the enzyme isolated from most animal sources (the one from muscle is the most
extensively studied), the basic side chain of an essential lysine residue plays
the key role in catalyzing this reaction. The thiol group of a cysteine also
acts as a base here.
Step 5. The dihydroxyacetone phosphate is converted to glyceraldehyde-3- phosphate.
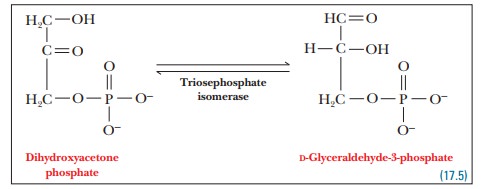
The
enzyme that catalyzes this reaction is triosephosphate
isomerase. (Both dihydroxyacetone and glyceraldehyde are trioses.)
One
molecule of glyceraldehyde-3-phosphate has already been produced by the
aldolase reaction; we now have a second molecule of glyceraldehyde-3-phosphate,
produced by the triosephosphate isomerase reaction. The original molecule of
glucose, which contains six carbon atoms, has now been converted to two
molecules of glyceraldehyde-3-phosphate, each of which contains three carbon
atoms.
The ∆G value for this reaction under physiological
conditions is slightly positive (+2.41 kJ mol–1 or +0.58 kcal mol–1).
It might be tempting to think that the reaction would not occur and that
glycolysis would be halted at this step. We must remember that just as coupled
reactions involving ATP hydrolysis add their G values together for the overall reaction, glycolysis is composed
of many reactions that have very negative G
values that can drive the reaction to completion. A few reactions in glycolysis
have small, positive ∆G values (see Table 17.1), but four reactions have very large,
negative values, so that the ∆G for the whole process is negative.
Summary
In the first stages of glycolysis, glucose is converted to two molecules
of glyceraldehyde-3-phosphate.
The key intermediate in this series of reactions is fructose-1,6-bisphosphate. The reaction that produces
this intermediate is a keycontrol point of the pathway, and the enzyme that
catalyzes it, phospho-fructokinase, is subject to allosteric regulation.
Related Topics