Chapter: Biochemistry: Glycolysis
Anaerobic Metabolism of Pyruvate
Anaerobic Metabolism of Pyruvate
How does the conversion of pyruvate to lactate take place in muscle?
The
final reaction of anaerobic glycolysis is the reduction of pyruvate to lactate.
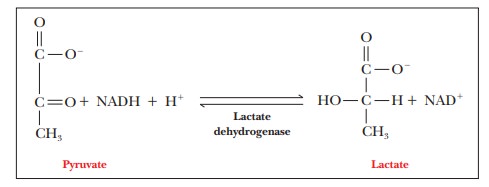
This
reaction is also exergonic ( ∆G°' = –25.1
kJ mol–1 = –6.0 kcal mol–1); as before, we need to
multiply this value by 2 to find the energy yield for each molecule of glucose
that enters the pathway. Lactate is a dead end in muscle metabolism, but it can
be recycled in the liver to form pyruvate and even glucose by a pathway called
gluconeogenesis (“new synthesis of glucose”).
Lactate dehydrogenase (LDH) is the enzyme that catalyzes this
reaction.Like glyceraldehyde-3-phosphate dehydrogenase, LDH is an NADH-linked
dehydrogenase and consists of four subunits. There are two kinds of subunits,
designated M and H, which vary slightly in amino acid composition. The
qua-ternary structure of the tetramer can vary according to the relative
amounts of the two kinds of subunits, yielding five possible isozymes. In human
skeletal muscle, the homogeneous tetramer of the M4 type
predominates, and in the heart the other homogeneous possibility, the H4
tetramer, is the predominant form. The heterogeneous forms—M3H, M2H2,
and MH3—occur in blood serum. A very sensitive clinical test for
heart disease is based on the existence of the various isozymic forms of this
enzyme. The relative amounts of the H4 and MH3 isozymes
in blood serum increase drastically after myocardial infarc-tion (heart attack)
compared with normal serum. The different isozymes have slightly different
kinetic properties due to their subunit compositions. The H4 isozyme
(also called LDH 1) has a higher affinity for lactate as a substrate. The M4
isozyme (LDH 5) is allosterically inhibited by pyruvate. These differ-ences
reflect the isozymes’ general roles in metabolism. The muscle is a highly
anaerobic tissue, whereas the heart is not.
At this
point, one might ask why the reduction of pyruvate to lactate (a waste product
in aerobic organisms) is the last step in anaerobic glycolysis, a path-way that
provides energy for the organism by oxidation of nutrients. There is another
point to consider about the reaction, one that involves the relative amounts of
NAD+ and NADH in a cell. The half reaction of reduction can be
written
Pyruvate
+ 2H+ + 2e– -
> Lactate
and the
half reaction of oxidation is
NADH + H+
- > NAD+ + 2e–
+ 2H+
The
overall reaction is, as we saw earlier,
Pyruvate
+ NADH + H+3 Lactate + NAD+
The NADH
produced from NAD+ by the earlier oxidation of
glyceraldehyde-3-phosphate is used up with no net change in the relative
amounts of NADH and NAD+ in the cell (Figure 17.11). This
regeneration is needed under anaerobic conditions in the cell so that NAD+
will be present for further glycolysis to take place. Without this
regeneration, the oxidation reactions in anaerobic organisms would soon come to
a halt because of the lack of NAD+ to serve as an oxidizing agent in
fermentative processes. The production of lactate buys time for the organism
experiencing anaerobic metabolism and shifts some of the load away from the
muscles and onto the liver, in which gluconeogenesis can reconvert lactate to
pyruvate and glucose. The same considerations apply in alcoholic fermentation
(which will be discussed next). On the other hand, NADH is a frequently
encountered reducing agent in many reactions, and it is lost to the organism in
lactate production. Aerobic metabolism makes more efficient use of reducing
agents (“reducing power”) such as NADH because the conversion of pyruvate to
lactate does not occur in aerobic metabolism. The NADH produced in the stages
of glycolysis leading to the production of pyruvate is available for use in
other reactions in which a reducing agent is needed.
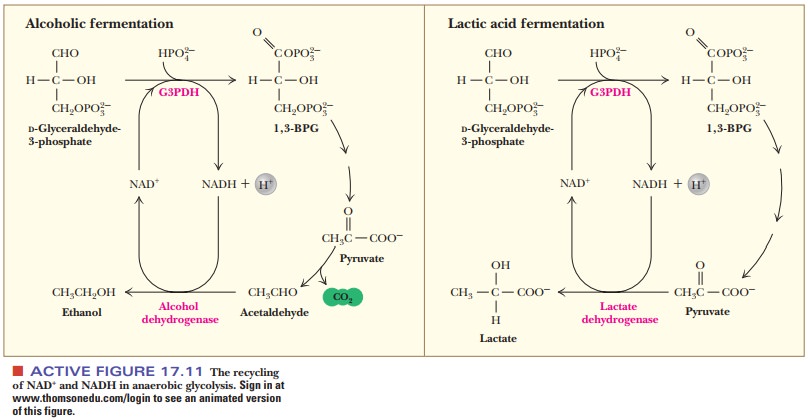
How does alcoholic fermentation take place?
Two
other reactions related to the glycolytic pathway lead to the production of
ethanol by alcoholic fermentation.
This process is one of the alternative fates of pyruvate. In the first of the
two reactions that lead to the production of ethanol, pyruvate is
decarboxylated (loses carbon dioxide) to produce acetaldehyde. The enzyme that
catalyzes this reaction is pyruvate
decarboxylase.
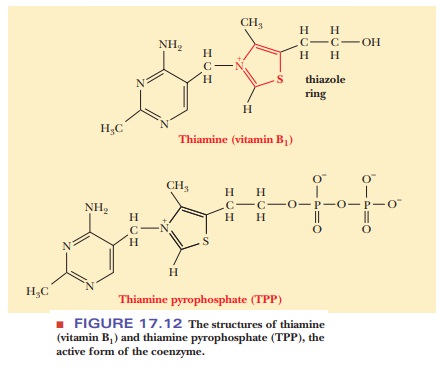
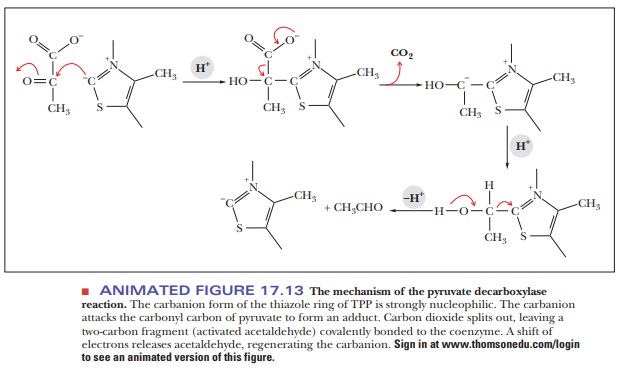
This
enzyme requires Mg2+ and a cofactor we have not seen before, thiaminepyrophosphate (TPP). (Thiamine
itself is vitamin B1.) In TPP the carbon atombetween the nitrogen
and the sulfur in the thiazole ring (Figure 17.12) is highly reactive. It forms
a carbanion (an ion with a negative charge on a carbon atom) quite easily, and
the carbanion, in turn, attacks the carbonyl group of pyruvate to form an
adduct. Carbon dioxide splits off, leaving a two-carbon fragment covalently
bonded to TPP. There is a shift of electrons, and the two-carbon fragment
splits off, producing acetaldehyde (Figure 17.13). The two-carbon fragment
bonded to TPP is sometimes called activated acetaldehyde, and TPP can be found
in several reactions that are decarboxylations.
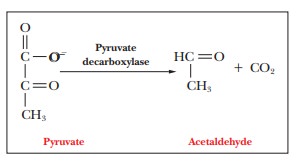
The
carbon dioxide produced is responsible for the bubbles in beer and in sparkling
wines. Acetaldehyde is then reduced to produce ethanol, and, at the same time,
one molecule of NADH is oxidized to NAD+ for each molecule of
ethanol produced.
Acetaldehyde
+ NADH - > Ethanol + NAD+
The
reduction reaction of alcoholic fermentation is similar to the reduction of
pyruvate to lactate, in the sense that it provides for recycling of NAD+
and thus allows further anaerobic oxidation (fermentation) reactions. The net
reac-tion for alcoholic fermentation is
Glucose
+ 2ADP + 2Pi + 2H+ - > 2 Ethanol + 2ATP + 2CO2
+ 2H2O
NAD+ and NADH do not appear explicitly in the net equation. It is essential that the recycling of NADH to NAD+ takes place here, just as it does when lactate is produced, so that there can be further anaerobic oxidation. Alcoholdehydrogenase, the enzyme that catalyzes the conversion of acetaldehyde toethanol, is similar to lactate dehydrogenase in many ways. The most striking similarity is that both are NADH-linked dehydrogenases, and both are tetramers.
Summary
Pyruvate
is converted to lactate in anaerobic tissues, such as actively metabolizing
muscle. NAD+ is recycled in the process.
In some
organisms, pyruvate is converted to ethanol in a process requir-ing thiamine
pyrophosphate as a coenzyme.
Related Topics